Summary1
Conventional reconstructive surgery can provide impactful results for many patients but can be limited in what it can do for persons with catastrophic tissue destruction or loss due to trauma, tumors, sepsis, or congenital conditions, leaving some of these patients with poor functional and aesthetic outcomes and severe psychosocial distress. Now, vascularized composite allotransplantation (VCA) 2 offers an innovative therapeutic option for some of these patients. VCA involves the transplantation of a body part that contains multiple tissue types (e.g., skin, fat, muscle, bone, nerves, and blood vessels) to a recipient as a functional unit (e.g., a hand or face) to restore and recreate the anatomy of the original body part. Currently, VCAs includes face, hands/upper limbs, abdominal wall, uterus, penile, scalp, larynx, and trachea.3 VCA is generally considered life-enhancing, achieving restoration of tissue defects with improved functional and aesthetic outcomes and allowing patients to regain bodily functions and greater independence.
Among the multiple types of VCA, the most experience exists for face and hand transplantation. Over the past two decades, there have been approximately 53 face transplants and 151 unilateral or bilateral hand
___________________
1 This summary does not include references. Citations to support the text and recommendations herein are provided in the main text.
2 Depending on the context, VCA may also stand for vascularized composite allograft, and VCAs for vascularized composite allografts.
3 The focus of this report is face and hand (upper limb) transplants.
transplants.4 These two types of VCA have demonstrated technical success and long-term survival for numerous patients. The surgical, immunological, and functional feasibility of these treatments has been established over the past three decades, and the available evidence shows that they are a viable therapeutic option for select patients. However, notwithstanding their more than 20-year history, face or hand transplants are not routinely offered to potential recipients, and many challenges need to be addressed. These challenges and considerations include unique ethical and psychosocial issues, the need for lifelong immunosuppressive treatment and the associated hazards, uncertainty regarding the optimal transplant candidates, lack of sustainable funding, inadequate outcome measurement tools, and sparse longitudinal outcomes data.
Face and hand transplants are highly complex procedures associated with significant risks, and some important scientific questions about these transplants remain unanswered. Only a few transplant centers in the United States have the requisite infrastructure and other qualifications to perform and provide comprehensive high-quality face and hand transplantation care. However, even among this small group of transplant centers, there are no standardized protocols or validated clinical tools for patient selection, choice of surgical procedures, immunosuppression, rehabilitation, patient outcome measurement, or psychosocial support. The lack of standardized protocols makes it difficult to evaluate outcomes in a systematic fashion, especially given the small number of patients. There is a critical need to standardize, assess, and validate clinical protocols, standard operating procedures, and data reporting and collection for face and hand transplants.
STUDY SCOPE AND CONTEXT
The Department of Defense Reconstructive Transplant Research Program (RTRP) has long supported VCA research in the United States. As part of a larger clinical network award, RTRP commissioned a consensus study from the National Academies of Sciences, Engineering, and Medicine (National Academies) to provide formal findings and recommendations to guide the development of standard operating procedures for face and hand transplantation (see Box S-1 for the statement of task). The purpose of the larger clinical network award was to promote a major multi-institutional network of VCA centers and associated collaborators for the purpose of standardizing clinical protocols for face and hand transplantation and
___________________
4 As of December 2024. The number of face and hand transplants was calculated by recipient who received a transplant. Thus, a bilateral hand transplant is not counted as two transplants. However, a re-transplant is treated as a new transplant and therefore counted as an additional transplant in the total amount, despite the transplant being performed on the same recipient.
BOX S-1
Statement of Task
An ad hoc committee of the National Academies of Sciences, Engineering, and Medicine will conduct a study to advise the Reconstructive Transplant Research Program (RTRP) Clinical Network—and the broader vascularized composite allotransplantation (VCA) community—on principles and strategies for the standardization, assessment, and validation of protocols and/or standard operating procedures (SOPs) for both face and hand transplantation, with consideration of the following focus areas:
- Patient inclusion/exclusion criteria,
- Patient education,
- Surgical procedures,
- Rehabilitation,
- Immunosuppression and/or immunoregulation,
- Outcome metrics,
- Quality of life measures, and
- Patient reporting (e.g., registry).
Specifically, the committee will:
- Articulate principles and a framework—including ethical and psychological considerations—to guide the development of protocols and SOPs, drawing on the appropriate expertise, including scientific, medical, human subjects protection, and regulatory;
- Describe fair and equitable processes through which the RTRP Clinical Network could develop, review, revise, and finalize VCA protocols and SOPs for both face and hand transplantation;
- Describe considerations for mitigating and resolving conflicts that may arise during this phase of the development process; and
- Make recommendations for other actions the RTRP Clinical Network could take to ensure responsible, ethical, scientifically informative and clinically effective application of face and hand transplantation.
In developing the principles, framework, and processes to guide the development of protocols and SOPs for face and hand transplantation, the committee will take into account research that is relevant to active duty service members, veterans, military beneficiaries, and/or the American public; the landscape of regulations and policies under which these transplants occur; and ethical standards and values. The committee is not expected to develop specific protocols or SOPs.
The committee will prepare a report with its findings and recommendations.
assessing those protocols in multi-institutional clinical trials. This resulted in the creation of the Clinical Organization Network for Standardization of Reconstructive Transplantation (CONSORT).5 CONSORT has an opportunity to standardize face and hand transplantation; to address unmet needs, including the collection of outcomes data and the standardization of outcome measures; to answer persistent questions about optimal immunosuppressive management and patient selection criteria; to standardize the informed consent process; and to improve the pre- and posttransplant care of patients who could benefit from VCA. During the time that this study was undertaken, CONSORT worked in tandem to establish the clinical network and undertake other activities in preparation of the release of the National Academies report. All institutions that join CONSORT as “network sites”6 agree to participate in the development of standardized protocols and agree to follow the standardized protocols once finalized.
The committee consisted of 12 experts in the areas of VCA surgery, immunosuppression, patient management, bioethics, health care law and policy, psychology/psychiatry, rehabilitation, shared decision making, patient outcomes, biostatistics, and solid organ transplantation. In addition to the committee member expertise, additional input was obtained through three major mechanisms: (1) a series of public information-gathering webinars; (2) input from face and hand transplant recipients and a caregiver, who were appointed as lived experience consultants to the study and met with the committee to discuss their transplant experience and provided comments on draft versions of the report; and (3) a call for perspectives on the study website. Quotes and information expressed through these mechanisms are used throughout the report. Additionally, the committee performed a comprehensive literature review of the peer-reviewed and gray literature.
The committee has provided findings, conclusions, and recommendations related to the eight focus areas listed in the statement of task. The committee has also developed recommendations to guide CONSORT on developing fair and equitable protocols and standard operating procedures (SOPs) and other actions to ensure responsible, ethical, safe, scientifically informative, and clinically effective application of face and hand transplantation. This report does not develop the clinical protocols and standard operating procedures for face and hand transplantation but rather provides the evidence, principles, and a framework to guide their development. Additionally, while the committee heard from international experts and consulted international literature, the primary focus of this report is on transplants occurring within the United States, which has a health care
___________________
5 See https://consortrial.org/ for more information (accessed October 13, 2024).
6 As of December 26, 2024, eight transplant centers have joined CONSORT as network sites.
landscape of regulatory, financing, and legal policies that significantly differ from other countries that have performed or will perform these transplants. Finally, recommendations related to payment and issues related to health insurance coverage, while critical to the future of face and hand transplantation, were not a focus of the committee’s deliberations nor of this report.
BACKGROUND ON VCA
The Organ Procurement and Transplantation Network (OPTN) Final Rule,7 which established a regulatory framework for the structure and operations of the OPTN, states that all U.S. centers that perform organ transplants are required to submit certain data—including patient demographics, wait times for a donor organ, blood types, donor authorization requirements, and clinical outcomes—for the purposes of promoting safety and improving patient outcomes. VCAs have been included under the Final Rule since 2014, and VCA programs must meet specific requirements to be approved by the OPTN. As of December 2024, there were 10 OPTN-approved sites for head and neck VCA procedures and 11 approved sites for upper limb transplants. However, even OPTN-approved sites face diverse challenges. For example, VCA donor authorization presents challenges that differ from other transplants, including that many donation professionals do not have experience discussing with families the unique issues related to VCA donation (e.g., concerns related to funeral services for the potential donor). There also is a lack of public awareness regarding VCA and its different types.
Ethical challenges for face and hand transplantations center on autonomy and consent (e.g., informed consent, shared decision making, awareness of alternative options, and consent for life-enhancing versus lifesaving procedures); beneficence for vulnerable patients (e.g., privacy and vulnerability concerns, psychosocial benefits, long-term monitoring and care management); nonmaleficence (e.g., surgical risk, need for more research); and justice, both in access and eligibility (e.g., patient selection and discrimination, geographic access barriers, allocation of resources and availability of funding). Psychosocial factors, encompassing an individual’s mental health, coping skills, social support connections, and overall life circumstances, play a crucial role in optimizing medical and mental health outcomes. The recipients of hand and face transplants encounter complex psychosocial challenges related to the external visibility of these transplants and their identity, in terms of both first-person sense of self and in social interactions with others. The substantial financial burden for patients and families due
___________________
7 42 CFR Part 121.
to the costs of follow-up care and immunosuppression medications remains a challenge after institutional support ends.
THE TRANSPLANT EXPERIENCE
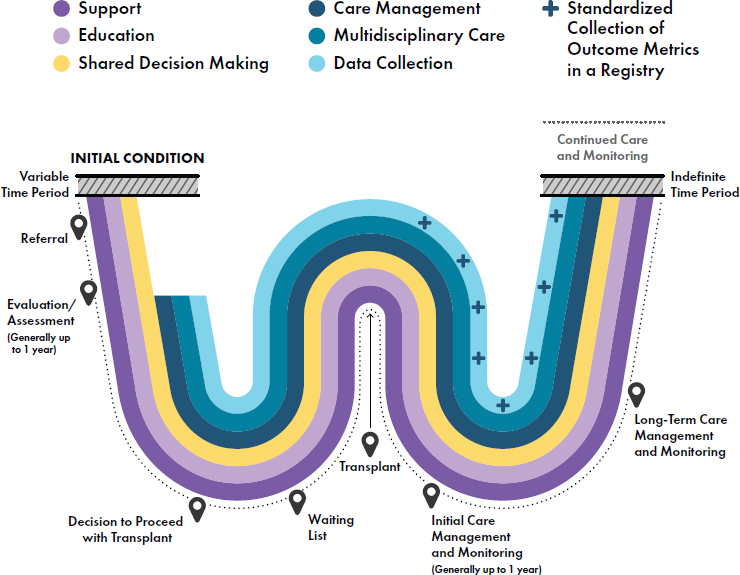
The transplant experience is extremely complex and life changing for both recipients and their caregivers. When referring to face and hand transplantation throughout the report, the committee emphasizes that this characterization does not solely refer to the surgical procedure, but instead to a whole health care approach to the longitudinal transplant experience that centers around the patient. Whole health care is defined as “an interprofessional, team-based approach anchored in trusted longitudinal relationships to promote resilience, prevent disease, and restore health. It aligns with a person’s life mission, aspiration, and purpose.”8 The transplant experience begins well before the surgery takes place and continues throughout the lifetime of the allograft and the patient. This whole health care approach encompasses all experiences that recipients and their caregiver(s) go through that are associated with the transplant. It also emphasizes the need for multidisciplinary teams that foster trust among clinicians, patients, and caregivers. The devastating tissue loss that results in the need for these procedures affects all domains of a person’s life, and a surgical procedure will not mitigate these issues without comprehensive care management. Thus, an all-encompassing approach—i.e., a whole health approach—is needed. Underpinning the whole health approach is education, shared decision making, multidisciplinary care, and lifelong support for both patients and caregivers. Care management, monitoring, and data collection are essential across the entirety of the transplant experience.
The transplant experience includes the preoperative stage, surgical procedures during transplant, and the initial and long-term care management, monitoring, and data collection of outcomes (see Figure S-1).
The Transplant Experience: The Preoperative Stage
Shared decision making9 is an essential component of the face and hand transplant experience. It is an important step in empowering patients and caregivers and facilitating patient engagement in achieving their health goals. A face or hand transplant is one treatment option to be considered
___________________
8 Definition found in NASEM. 2023. Achieving whole health: A new approach for veterans and the nation. Washington, DC: The National Academies Press.
9 Shared decision making is defined as “an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options, to achieve informed preferences.”
alongside alternative treatments through a shared decision-making process. However, at present, there is a lack of standardization regarding the information provided to patients, including the treatment choices that need to be considered for those who are contemplating these types of transplants, the risks of immunosuppression, privacy considerations, and the lifelong commitment to care management and monitoring required for these types of transplants.
Face and hand transplantation candidates should undergo a rigorous screening process, with both medical and psychosocial evaluations by a multidisciplinary team to assess their suitability for the procedure (e.g., immunological status, viral status, functional deficits, overall health, social support, and ability for self-reliance and adherence with directed care requirements following transplantation), including commitment to extensive rehabilitation, acceptance of and adherence to a strict, lifelong regimen of immunosuppressant medication to prevent rejection of the allograft, medical care management to assess the general health and viability of the transplant, and participation in regular assessments and follow-ups for
as long as the patient has the transplant. This evaluation process has not always been consistent or sufficiently comprehensive across transplant centers historically, which may have contributed to mixed and some marginal outcomes, particularly in the early years of VCA.
The needs of both patients and caregivers will likely be dynamic throughout the transplant experience, necessitating reassessment to adjust goals to align with the whole health approach to care. The assessment of candidates is best completed over a substantive period of time, often lasting a year or more. Pretransplant assessment allows for the timely identification and remediation of coping challenges; optimization of medical, surgical, immunological, and rehabilitation care plans; appropriate identification and addressing of biases such as social desirability; and identification of other needs, all of which can improve the likelihood of a successful transplant. In addition to the time needed for the potential recipients and their families to fully appreciate the commitment required before making the decision to proceed with transplant, the extended time required for evaluation also gives time for the surgical team to prepare for the unique needs of each recipient and to build needed trust and rapport with both patient and caregiver.
The Transplant Experience: Surgical Procedures and Care Management
After a comprehensive pretransplant assessment process, the next stages of the experience include the surgical procedures, immunosuppression, rehabilitation, and psychosocial management. The surgery itself requires extensive planning and coordination, including surgical rehearsals, use of advanced technologies (e.g., computerized surgical planning and cutting guides), coordination with organ procurement organizations, and development of a surgical exit plan. Surgical procedures and techniques are fairly standardized across centers, but the details of each transplant are dependent on the level of injury, facial deficit, or amputation. After surgery, recipients require immunosuppressive medications for the life of the transplant to mitigate the high risk of transplant rejection. A standardized approach to immune management of VCA recipients has not been established. Additional research is needed to establish the principles that should underlie a VCA-specific immunosuppression regimen that both prevents rejection and minimizes immunosuppressant drug-related morbidity. While patients will likely need individualized rehabilitation approaches depending on their initial injury, there are core components across rehabilitation protocols that can be used across transplant centers. Innovation and adaptability in rehabilitation protocols can still be explored within these standardized protocols.
Psychosocial support focuses on helping patients integrate their new face and/or hand(s)/upper extremities into their identity and navigate the psychological challenges that arise. Caregivers help patients navigate all aspects of pre- and posttransplant life. Regrettably, the needs of caregivers often have been overlooked in the past. Ongoing evaluation ensures that candidates, recipients, and caregivers receive the necessary support to navigate the challenges and maximize their well-being throughout the transplant experience.
The Transplant Experience: Outcomes
The outcomes that patients experience following hand and face transplantation extend far beyond the simple medical or surgical technical metrics of success or failure. However, there is a lack of consensus on the most important outcomes for face and hand transplantation (and particularly about what patients perceive as the most important outcomes), and no standardized set of outcome metrics has been defined or consistently used. Validated functional outcome measurements are critical for the continued maturation of face and hand transplantation and clinical care, and measurement instruments that have content validity along with reliability are essential. Both generic and condition-specific health-related quality of life (HRQoL) measures are necessary for accurate assessment that is sufficiently comprehensive, though the small patient population will likely affect validation of these measures.
A FRAMEWORK FOR STANDARDIZING FACE AND HAND TRANSPLANTATION
Vascularized composite allotransplantation is a frontier in transplantation, with face and hand transplants currently being the most developed parts of that frontier. For over two decades, face and hand transplantation practitioners and centers have worked to develop proven and safe surgical techniques, but as detailed in this report, many challenges remain throughout the transplant experience. With those challenges in mind, the committee espouses a framework for the future of face and hand transplantation (see Figure S-2) that builds upon the cross-cutting dimensions of value, transparency, and equity. This framework and the cross-cutting dimensions are based upon the 2022 National Academies report Realizing the Promise of Equity in the Organ Transplantation System and the 2010 Institute of Medicine report Future Directions for the National Healthcare Quality and Disparities Reports. The major principles underlying this vision include multidisciplinary and comprehensive care, accountability, sustainable funding, ongoing investigation of clinically and scientifically undefined areas, patient/family centeredness, and team competency.
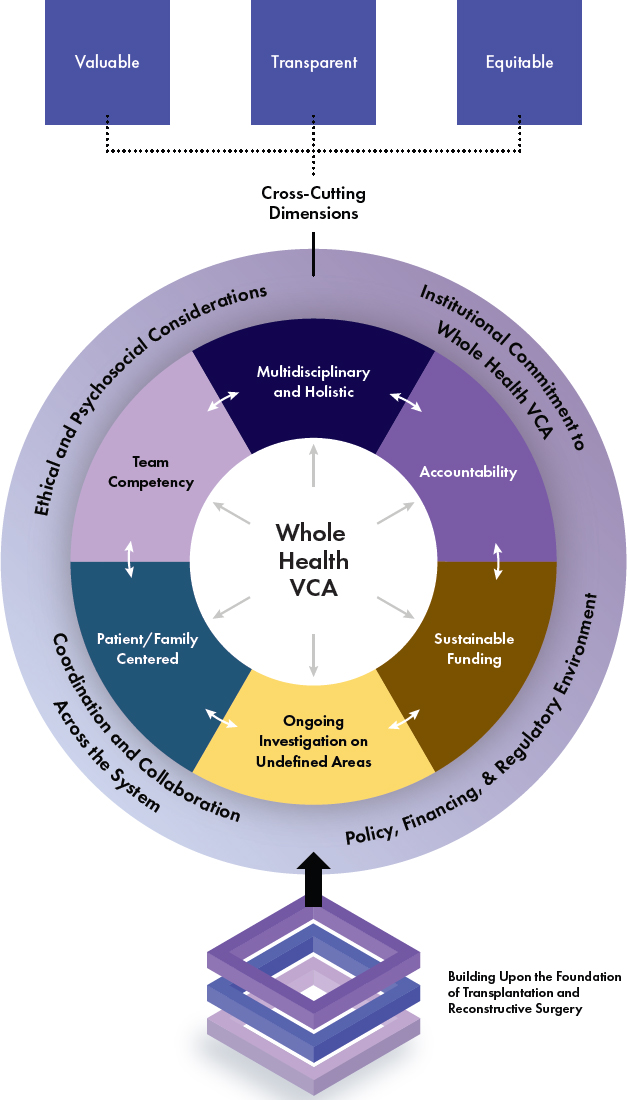
NOTE: VCA = vascularized composite allotransplantation.
The CONSORT initiative and collaboration among the broader VCA community are needed to standardize and normalize the face and hand transplantation experience. The VCA community must build upon its successes and failures, learn from other more established fields, and work collaboratively across institutions and disciplines, always keeping the central focus on the patient. CONSORT and all active VCA transplant centers have an obligation to adopt a comprehensive framework for ensuring the safety and well-being of individuals who undergo these procedures, including ensuring that recipients and their caregivers are adequately prepared for the VCA experience and are supported in ways that are likely to optimize treatment outcomes for as long as the recipient has the transplant. All VCA centers must emphasize the importance of optimizing high-quality care by committing to criteria of excellence, including being assiduous about informing patients of risks, benefits, alternatives, and complications and setting realistic expectations; providing the individualized support needed to optimize long-term outcomes, including interventions for psychosocial concerns; and otherwise minimizing the likelihood of suboptimal outcomes and critically evaluating and managing them when they occur. The community must also work together to document each transplant experience in a standardized way to inform future care.
OVERARCHING CONCLUSIONS AND RECOMMENDATIONS
The committee’s recommendations are organized around five overarching conclusions. The first is that face and hand transplantation are innovative, potentially transformative treatment options for certain patients when performed at select transplant centers that have experienced, multidisciplinary teams capable of reliably, ethically, and safely performing these procedures and providing ongoing, lifelong support to the recipients and their caregivers. Second, the committee concludes that the complexity of the face and hand transplant experience and the ongoing need for long-term care management for as long as the recipient has the transplant requires that a “whole health”10 approach be used for these transplant recipients and their caregivers. Additionally, and third, the committee concludes that face and hand transplantation would benefit from standardization of pretransplantation, procedural, and posttransplantation management and monitoring protocols, including how to assist patients and their caregivers in
___________________
10 Whole health is defined as the “physical, behavioral, spiritual and socioeconomic wellbeing as defined by individuals, families, and communities. To achieve this, whole health care is an interprofessional, team-based approach anchored in trusted longitudinal relationships to promote resilience, prevent disease, and restore health. It aligns with a person’s life mission, aspiration, and purpose.”
making informed decisions about whether to pursue these treatments. In furtherance of this goal, and fourth, the committee concludes that a single centralized, easily accessible, and adaptable patient registry is needed to collect and curate procedural and outcomes data that can then be analyzed to support operational needs, follow-up care, and further investigation. Fifth and finally, the committee concludes that active collaboration across all centers performing these transplants and ongoing research is crucial to further maturation of the scientific underpinnings of face and hand transplantation.
Based on these five overarching conclusions, the committee’s five recommendations are aimed at addressing three paramount needs:
- The need for a whole health approach to care provided at demonstrably high-quality transplant centers;
- The need for collaboration across the face and hand transplant community that will, among other things, facilitate needed standardization of care management, monitoring, and data management protocols used before transplantation, during the procedure, and post transplantation; and
- The need for a systematic approach to further investigation.
Whole Health Care Approach to Face and Hand Transplantation at Demonstrably High-Quality Transplant Centers
Applying a whole health approach to face and hand transplantation allows for all aspects of the transplant experience to be addressed in a comprehensive and supportive manner. Critical aspects of whole health VCA care include education, shared decision making, multidisciplinary clinical care, and lifelong support. Requiring all face and hand transplant centers to use a whole health approach and emphasize demonstrably high-quality care is vital to ensuring that patients are provided with appropriate multidisciplinary support, care management, and monitoring across all transplant centers. The complexity of the care required for recipients of face and hand transplants requires, in addition to the use of a whole health approach, that the transplants be performed at experienced centers capable of providing the highest quality of care. Aside from the OPTN requirements, there are currently no unified standards or criteria to promote high-quality VCA care across transplant centers. To standardize the quality of care across face and hand transplant centers, the committee recommends:
Recommendation I: In accordance with a whole health approach toward vascularized composite allotransplantation care and an emphasis on demonstrably high-quality care, transplant centers offering face and/or hand transplantation should commit to the following:
- Ensuring that formal ethical oversight processes and procedures are instituted and implemented;
- Establishing a multidisciplinary care team that includes the active participation of those with relevant expertise in transplantation, surgery, immunology, rehabilitation, mental health care, social work, pharmacy, and other relevant areas, as well as patient advocates and patient navigators;
- Using a comprehensive, dynamic, and longitudinal pretransplant assessment process for potential recipients that is undertaken by a multidisciplinary team, with the understanding that this process typically takes a year or so over multiple sessions with the exact time being patient-dependent;
- Creating a comprehensive patient and caregiver education program that supports shared decision making and includes an ongoing assessment of patient and caregiver understanding of all the aspects of the transplant experience;
- Implementing longitudinal posttransplant assessments to inform relevant medical, surgical, immunological, and psychosocial interventions. Caregivers should be included in assessments, enabling them to develop a comprehensive understanding of their own mental health needs, coping mechanisms, and resilience and to inform relevant interventions as needed; and
- Supporting and sustaining ongoing research.
Furthermore, there is no standardized education and training for clinicians interested in performing these transplants, and many clinicians who care for patients who may be suitable candidates for these transplants are often unaware of these options. Referral processes are neither standardized nor straightforward. To raise awareness of face and hand transplantation and improve overall face- and hand-transplant specific team competence, additional training standards are needed. Thus, the committee recommends that:
Recommendation II: Professional societies representing transplantation and reconstructive surgery professionals should collaborate to jointly develop and promulgate standards for the education and training of face and hand transplantation clinicians and communication strategies to increase awareness of these transplants as a treatment option.
Professional societies that may be best suited to participate in this effort include the American Society for Reconstructive Transplantation, the American Society of Transplantation, and the American Society of Transplant Surgeons. Given the number of face and hand transplants that
are performed outside of the United States, coordination with international societies such as the International Society of Vascularized Composite Allotransplantation and the Transplantation Society would be beneficial. Other relevant societies include the American Academy of Physical Medicine and Rehabilitation, the American Association for Hand Surgery, the American Association of Plastic Surgeons, the American Congress of Rehabilitation Medicine, the American Physical Therapy Association, the American Occupational Therapy Association, the American Psychological Association, the American Society for Plastic Surgeons, the American Society for Reconstructive Microsurgery, the American Society for Surgery of the Hand, and the Association of Academic Physiatrists.
Collaboration and Standardization
If face and hand transplantation is going to mature as other areas of transplantation have done, then the community broadly needs to adopt a strategy of active collaboration that prioritizes the needs of the specialty over those of individual institutions. Collaboration among the broader face and hand transplant community—U.S. and international face and hand transplant centers and their clinicians; basic science, translational, and clinical investigators; organ procurement organizations; funding agencies; patients and caregivers; and other community partners—will be necessary to standardize the transplantation experience and resolve outstanding clinical issues. Toward that end, the prioritization of developing and operationalizing a strategy of active collaboration aimed at achieving a systematic and standardized approach to the whole health transplantation experience is needed.
The CONSORT initiative has a unique opportunity to advance face and hand transplantation by working to standardize protocols and SOPs. To do so will require CONSORT to work in a transparent and accountable manner using a formal consensus development method analogous to what the health sector has done with developing performance measures over the past two decades. Figure S-3 depicts the committee’s visualization of the SOP development process, which builds upon a foundation of trust and relationship within the network, a review of the evidence, and a formal consensus development process. Communication both within and outside the clinical network should be consistent, timely, and transparent during this standardization process. Once standardized protocols are agreed upon, it will be important for leadership at transplant centers to commit to using them and to providing the institutional support necessary to ensure transplant team competence and expertise in this regard.
Other clinical networks have reported success from focusing on the following themes: patient involvement, data sharing internal and external
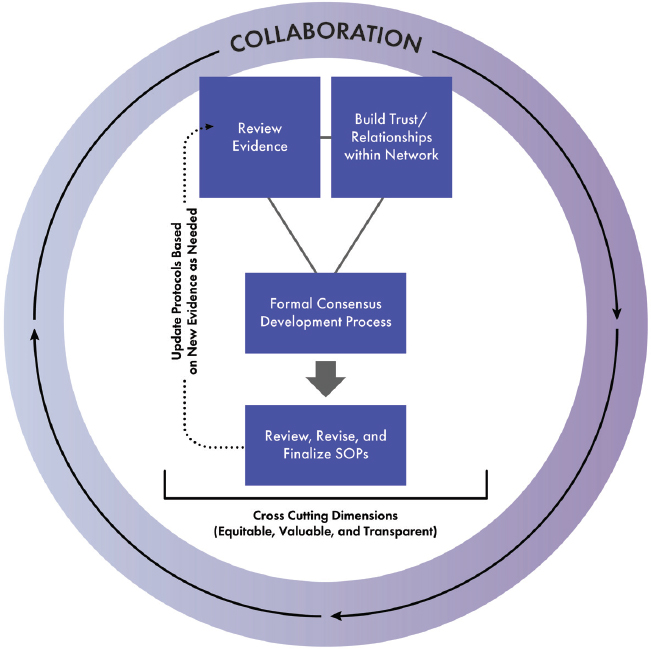
NOTE: SOPs = standard operating procedures.
to the network, transparency, rigorous and systematic data collection, effective leadership and streamlined open governance structure, inclusivity, education and training, centralized processes, well-delineated collaboration and authorship guidelines, continuous self-inspection, and programmatic involvement from the funding agency. To achieve the goals of CONSORT and concomitant with the committee’s focus on a whole health care approach to face and hand transplantation as laid out in Recommendation I, the committee recommends that:
Recommendation III: CONSORT should:
- Actively collaborate with the entire face and hand transplantation community and, more broadly, with the vascularized composite allotransplantation (VCA) community, including patients and caregivers.
- Use a formal consensus development method to standardize clinical protocols and operating procedures.
-
Prioritize the standardization of the following areas, while concomitantly ensuring that each area also has formal processes for ensuring continuous review for quality improvement purposes:
-
Shared decision making:
- Develop and implement a comprehensive, standardized education and shared decision-making process for patients, caregivers, and other community partners.
-
Immunosuppression regimens:
- Use immunosuppressive regimens that have been proven effective in other relevant clinical settings (e.g., solid organ transplantation) while making VCA-specific adaptations when supported by evidence and emerging data.
-
Data collection:
- Systematically collect medical, immunological, surgical, functional, and psychosocial outcomes using agreed-upon standardized format and outcome measures and measurement tools for all recipients, both before and following the transplant at predetermined timepoints, using both generic and transplant-specific measures as well as qualitative assessments.
- Specifically collect data, at a minumum on the indication for transplant; medical and surgical complications (e.g., metabolic, infectious, immunological); immunosuppressive regimens, rejection grade, and treatment of rejection; functional outcomes; posttransplant mental health and quality of life outcomes; re-transplantation; and death.
- Use strong, state-of-the-art data privacy protections.
-
Shared decision making:
- Develop a patient registry (database) that employs state-of-the-art, advanced data analytic capabilities and functionalities that will support and facilitate timely reporting and sharing of standardized outcome information.
- Engage with transplant centers that are interested in initiating or expanding hand and face transplantation programs, provided that the centers commit to the conditions specified in Recommendation I and work closely with more experienced centers to develop needed expertise.
- Create formal conflict resolution processes and timelines to ensure that each transplant center or individual, whether internal or external to the consortium, has an avenue to resolve conflict expeditiously.
To build trust and to support sustainable relationships within the network and broadly within the transplant community, CONSORT should be transparent about which consensus development method it chooses and clearly articulate how and for what purposes the methodology will be used. Similarly, its formal conflict mitigation methods should address conflict arising at all levels and specify timelines for the resolution of issues. The education and shared decision-making plan should include an evidence-based decision-aid tool that can be used by patients, caregivers, and clinical teams and that includes information about the transplant experience; alternative therapeutic options; the risks and complications of the surgery and of immunosuppression; and the lifelong commitments required for face and hand transplant recipients, their caregivers, and the corresponding institution. The decision tool should include exercises to clarify values and goals to allow patients and caregivers to express their priorities. The standardized data collection should include time-points for routine surveillance and biobanking of the study protocol and for-cause samples of blood, serum, and tissue biopsies for further investigation. Additionally, data collection should include assessments using the standardized Banff VCA classification system for rejection. The registry should build upon the work of the Scientific Registry of Transplant Recipients and the International Registry on Hand and Composite Tissue Transplantation. In addition to data on patients who receive a transplant, the registry should also include information on all candidates who are evaluated for face and hand transplantation, including outcomes data as available. The longitudinal data collection should be led by an experienced data scientist with experience in small study populations and observational data. This registry should also share the data with those not involved in CONSORT, in alignment with data sharing agreements. When considering the inclusion of international data in the registry, given the differing protocols and data collection methods used around the world and to maintain the highest possible data quality of the registry, it may be beneficial to take a country-by-country approach, with an eye toward confirming the validity of any submitted data that were not collected under the standardized protocols. Harmonization should be strived for given the numbers of these transplants that are performed outside of the United States and the recognition of the importance of international collaboration.
The committee believes that it is critical that CONSORT receives ongoing external scientific advice and oversight. While there may be existing advisory committees within RTRP, the committee believes that CONSORT
needs additional oversight and advice regarding face and hand transplantation specific to CONSORT. To this end, the committee recommends that:
Recommendation IV: The Reconstructive Transplant Research Program should establish an independent, multidisciplinary committee that is empowered to provide ongoing external oversight and scientific advice to CONSORT. Responsibilities of this oversight committee should include:
- Providing ongoing guidance about the development of standardized protocols and standard operating procedures and issues involving conflict resolution, particularly in the early stages of network development when more frequent advice may be needed;
- Providing other advice as needed to appropriately embed the committee’s framework and principles within the clinical network; and
- Providing at least annual evaluation and review of the network’s progress toward its goals and the scientific merit of its research program.
The relevant expertise that should be included on this committee includes transplant surgery and immunology, rehabilitation, mental health care, and social work as well as expertise in program evaluation, data management, health policy, bioethics, and representation of the patient and caregiver perspective.
Systematic Approach to Continued Investigation
For maturation of face and hand transplantation, there must be ongoing research on all aspects of the transplant experience. One example, among many, of where such research would be valuable can be found in the lack of generalizable psychosocial, surgical, or medical criteria among VCA candidates and recipients. Similarly, while there are common components across rehabilitation protocols, there is a need for innovation, adaptability, and additional research in rehabilitation techniques and regimens for VCA recipients. There is not an agreed-upon, generalizable approach to immune management. Additional research is needed to establish the principles underlying a VCA-specific immunosuppression regimen and to develop immunosuppressive regimens for face and hand transplantation that are more effective, less costly, more easily tolerated, and safer. Likewise, validated functional outcome measurements are needed. Measurement instruments having content validity and reliability specifically for VCA are also needed. The engagement of an established consensus standards and
quality measurement body (e.g., the National Quality Forum) could be helpful in this regard. The committee recommends that:
Recommendation V: The Reconstructive Transplant Research Program, National Institutes of Health, and other funders should continue to support research in face and hand transplantation specifically and vascularized composite allotransplantation (VCA) broadly. Topics that warrant priority consideration for further investigation include, but are not limited to:
- Immunosuppression and immunomodulation, focusing especially on evolving, effective immunosuppressive-based regimens that block specific alloimmune pathways to prevent rejection and on the development of donor-specific antibodies, and that avoid associated end-organ toxicities and other complications;
- Differential diagnoses of rejection, coexisting pathological diagnosis, molecular diagnosis, and surrogate endpoints (e.g., biomarker-based tools);
- Development of comprehensive patient- and family-centered VCA outcome measurement tools (e.g., functional, psychosocial, health-related quality of life) and evaluation of general population measures;
- Shared decision making;
- Patient selection, including optimal time to perform the transplant after initial injury; and
- Rehabilitation techniques and regimens.
Face and hand transplantation, as well as VCA broadly, are at a pivotal stage of development. Now is the time to collaboratively move forward, with a focus on whole health care and a systematic approach to continued investigation. The committee hopes that its recommendations are implemented, and that individuals and institutions involved with face and hand transplantation remain committed to unifying, standardizing, and improving protocols and standard operating procedures to achieve the best possible care for patients and caregivers.
This page intentionally left blank.



















