Charting a Path Toward New Treatments for Lyme Infection-Associated Chronic Illnesses (2025)
Chapter: Summary
Summary1
Tens of thousands of Americans develop Lyme infection-associated chronic illnesses (IACI) each year, a condition that can occur after acquiring Lyme disease and that often presents as debilitating physical symptoms including chronic fatigue, recurring pain, cognitive dysfunction such as “brain fog,” and sleep disturbances despite antibiotic treatment for Lyme disease. Approximately 10–20 percent of the 476,000 individuals who develop Lyme disease each year following the bite of a tick infected with Borrelia burgdorferi, a spirochete bacteria, go on to develop Lyme IACI, which can lead to strains on interpersonal relationships, loss of careers, depression, anxiety, and other mental health conditions. People living with Lyme IACI also report being disbelieved by clinicians if objective clinical or laboratory findings are not able to substantiate the symptoms that they are experiencing. While some individuals eventually find relief through various interventions or return to functionality without treatment, many suffer from the condition for years. Much about Lyme IACI remains unknown. While there is a standardized diagnosis and treatment for Lyme disease, there are gaps in understanding the cause, diagnosis, and treatment for the persistent symptoms associated with Lyme IACI. Despite the need to better understand the disease, it is clear that Lyme IACI is debilitating to the health and well-being of tens of thousands of Americans. There is an urgent need for research to provide safe and effective treatments for Lyme IACI
___________________
1 This summary does not include references. Citations for the information presented herein are provided in the main text.
that address the symptoms that affect the functionality and quality of life of those living with the condition.
There are no available treatments validated to safely and effectively cure or manage symptoms for those living with Lyme IACI. As a result, affected individuals may rely on word of mouth or trial and error in the hopes of relieving their symptoms with interventions of unknown safety and effectiveness. While clinicians have worked to better understand and treat Lyme IACI, research to develop and evaluate therapeutic interventions has received insufficient attention and investment, partially due to a lack of alignment on research priorities. To find better treatments for Lyme IACI, both increased research, and collaboration and coordination among researchers, clinicians, and patient communities are critical.
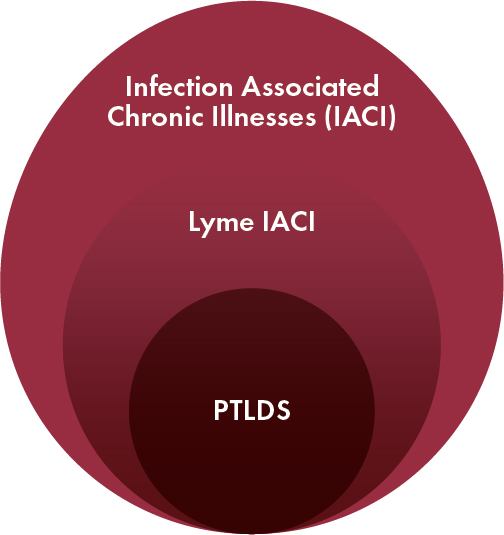
The broad recognition of Long COVID—a chronic condition associated with SARS-CoV-2 infection—has galvanized acceptance of Lyme IACI and promoted awareness that Lyme IACI and Long COVID are just two examples of chronic disease states with potential infectious triggers, referred to throughout this report as infection-associated chronic illnesses (IACI). The common threads among IACI are the similar chronic symptoms and the potential connection to an infectious trigger. These commonalities complicate the task of distinguishing individuals with Lyme IACI from individuals with any other IACI. Moreover, Lyme IACI has not been well defined as a condition as opposed to other IACIs such as Long COVID, though a subset of individuals who overlap with the Lyme IACI population has been defined as post-treatment Lyme disease syndrome (PTLDS) (Figure S-1). The population of people with Lyme IACI is heterogeneous: individuals with Lyme IACI differ in terms of symptom type and severity, and certainty of previous B. burgdorferi exposure. At the same time, the similarities among these various conditions present an opportunity, as researchers consider whether IACI may also share the same or similar underlying biological drivers or pathways. Until recently, however, there have not been concerted efforts to investigate these possible commonalities and translate knowledge across the silos of different disease areas to advance new treatments for Lyme IACI.
To address the lack of safe and effective treatments for Lyme IACI and to take advantage of the recent research advances stimulated by Long COVID, the Steven & Alexandra Cohen Foundation asked the National Academies of Sciences, Engineering, and Medicine (the National Academies) to assess the current evidence base for treatment of Lyme IACI and to identify priorities and new opportunities to advance treatment and diagnosis of this syndrome. Building on the recommendation in the 2022 report of the Tick-borne Disease Working Group, which called for a National Academies study to conduct a review on the basic science and clinical evidence for diagnosis and treatment that establishes “what is definitely known, what is partially understood, and what remains unknown”
for Lyme disease (covering early disease to the persistent symptoms), the current report reviewed the available evidence for treatment of Lyme IACI and for other similar conditions to illuminate a path toward advancing new treatments for Lyme IACI. An ad hoc committee consisting of experts in treating persistent symptoms associated with Lyme disease or similar conditions, clinical trials design and methodology, public health and epidemiology, neuroscience and infectious diseases research, health policy, medical ethics, community engagement, and individuals with lived experience with lingering symptoms associated with Lyme was assembled to respond to this request. To address this task, the committee reviewed the published literature on treatments for Lyme IACI and related conditions as well as the literature on the etiology and diagnosis of Lyme IACI. In addition to literature review, the committee carefully considered the perspectives shared by people affected by Lyme IACI and sought input from clinicians and researchers working to address Lyme and other IACI. The committee also gathered additional information through public presentations from researchers, technology developers, regulators, patients, and patient-led research organizations. A full discussion of the committee’s approach to this study in provided in Chapter 1.
The recommendations in this report seek to address gaps in the evidence base for Lyme IACI treatments and diagnosis and can be classified into four themes: centering the lived experience, shifting to treatment research that focuses on addressing symptoms, broadening the evidence base to systematically draw lessons from similar syndromes, and developing a unifying vision for future research. Additional considerations for the implementation of the report’s recommendations are discussed in Chapter 5.
PERSON-CENTERED, SYMPTOM-BASED TREATMENT DEVELOPMENT
In the absence of validated interventions for treating Lyme IACI, people living with this debilitating condition may try interventions that have not been adequately tested for safety or efficacy in the Lyme IACI population. The use of an untested treatment can put individuals living with Lyme IACI at risk of spending money on an ineffective product and of serious, unknown harm, particularly when multiple products are used in combination. Adults with capacity have the right to make their own health care decisions in consultation with a clinician. To support informed decision making, the efficacy, potential risks, benefits, and burdens of treatment need to be made clear through rigorous clinical evaluations. The discovery and development of safe and effective treatments has been slowed by the poor understanding of the biological mechanisms that lead to Lyme IACI. Ideally, curative treatments would be designed based on well-established disease mechanisms. However, full understanding of a disease’s etiology is not a prerequisite for research and development on effective interventions that target what is known: symptoms that have uprooted people’s lives. In the absence of clear mechanistic targets, it is imperative to pursue evidence-based interventions to relieve Lyme IACI symptoms and improve patients’ quality of life, while continuing targeted mechanistic studies to uncover the underlying disease mechanisms and better target and design future treatments.
To move toward this goal, it is essential to adopt patient-centric approaches, by partnering with individuals with lived experience for their input in defining and executing research priorities for Lyme IACI. Their valuable insights on the disease experience can help in the formulation of research questions that address real-world impact, guide the overall research agenda, and inform the research strategy. Moreover, there are many patient-led groups and research initiatives interested in providing input, but their knowledge and networks are currently underused. Igniting this engagement has multiple benefits for people living with Lyme IACI, researchers, and funders, including improvement of research translation to clinical care, increased recruitment and retention of study participants, and
more widespread dissemination of research findings. To promote ongoing patient-centeredness of Lyme IACI research, it will be necessary to seek and incorporate the input of individuals living with Lyme IACI in the implementation of the report’s recommendations.
SHIFTING THE FOCUS: RIGOROUS CLINICAL TRIALS THAT TARGET SYMPTOMS
To review the current state of knowledge regarding clinical trials for the treatment of Lyme IACI, the committee conducted a scoping review of the published research to identify current evidence gaps and key questions to be addressed in future research. The scoping review also included existing literature on diagnosis and disease mechanisms since findings from these two research areas can inform treatment research. The scoping review excluded unpublished data, some of which are contained within patient registries and biobanks. However, these data are valuable, and the report addresses strategies to improve their use in research. Given the current state of the evidence, the committee identified several overarching questions relevant to planning future research and maximizing opportunities for success (Box S-1). Where supported by the available evidence, the committee has begun to address these questions throughout the report but acknowledges that researchers and funders will need to continue to grapple with these questions as the future of Lyme IACI research is charted.
The scoping review revealed 19 clinical trials that had been conducted for treatments of Lyme IACI, compared with 49 studies on disease mechanism (Figure S-2). In general, the number of publications related to potential disease mechanisms or diagnoses of Lyme IACI steadily grew in the past two decades, while the number of randomized controlled clinical trials for treatment stagnated despite the need for additional research. Many challenges have slowed progress in developing Lyme IACI treatments, including
the limited understanding of disease mechanisms that could guide the development of therapeutics.
Paucity of Rigorously Conducted Clinical Trials on Potential Treatments
Of the 19 publications on potential treatments for Lyme IACI, only five were randomized, controlled, and blinded clinical trials, and all five of those trials evaluated the effects of extended antibiotics regimens. A sixth randomized, controlled trial, which was not blinded, tested the benefit of yoga for Lyme IACI (Figure S-2). Taken together, the six randomized trials did not find consistent evidence of sustained benefits, and the antibiotic trials demonstrated some adverse effects. There have not been any randomized trials conducted in pediatric populations. The remaining 13 clinical trials evaluated a wider range of interventions, from antibiotics to electromagnetic radiation, exercise, immunosuppressants, dietary interventions, nutritional supplements, and cognitive behavioral therapies. However, these trials lacked important elements of rigorous study design, such as randomization or controls. Without rigorous methods in the study design, it is not possible to assess the scientific validity of the studies’ outcomes.
Whether different approaches to the use of antibiotics could provide a benefit remains a point of scientific debate, but given the poor understanding of the many potential biological mechanisms in Lyme IACI, the near exclusive focus of the Lyme IACI research portfolio on antibiotic therapies lacks a clear scientific rationale. Antibiotics are only likely to be effective in treating Lyme IACI if the driver of symptoms were the persistence of Borrelia spp. despite the initial treatment of the infection. However, the pathogen persistence hypothesis is only one of many that have been proposed as the mechanism of chronic symptoms. Others include autoimmunity, central nervous system dysfunction, antigen persistence, metabolic changes, or microbiome changes. Given the current limited understanding of Lyme IACI mechanisms, it is premature to focus the conduct of randomized trials on interventions that target a single mechanism at the exclusion of others.
Moving Forward with Clinical Trials for Improving Symptoms
Ideally, knowledge of Lyme IACI disease mechanisms will make it possible to carry out more targeted research and development for treatments that act on specific disease pathways to alleviate symptoms or eventually find a cure. But as long as this knowledge is not available, it is critical to explore additional approaches to develop potential interventions that can address symptoms and improve people’s quality of life even if the underlying disease process is not yet fully understood. Testing potential therapeutic
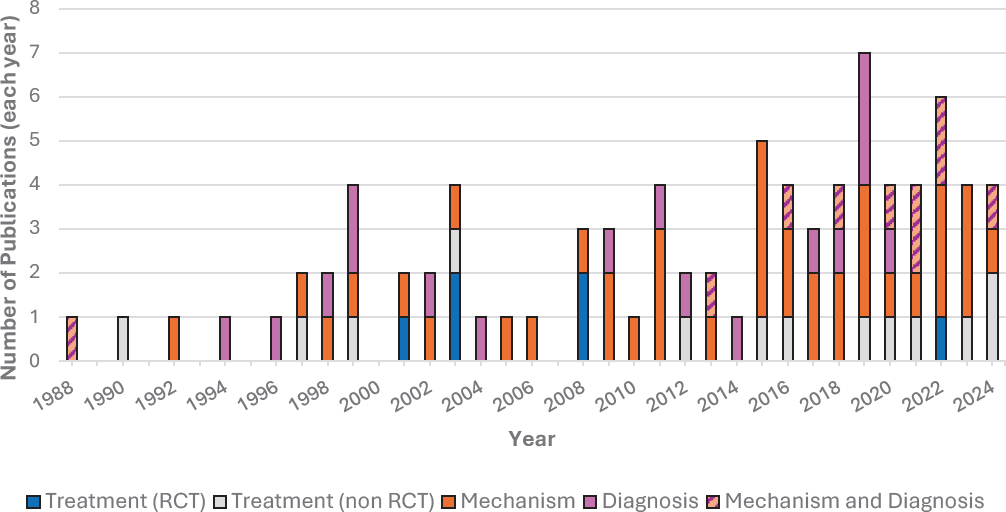
NOTE: IACI = infection-associated chronic illnesses; RCT = randomized controlled trial.
candidates to address Lyme IACI symptoms takes advantage of the existing evidence that can be drawn from other conditions with similar symptoms and the lived experience from individuals with this condition. There are many potential Lyme IACI treatments for which additional research may be warranted, including some currently in use by those living with the condition. Other potential treatments may be proposed by researchers and clinicians based on scientific evidence from Lyme IACI or other similar conditions.
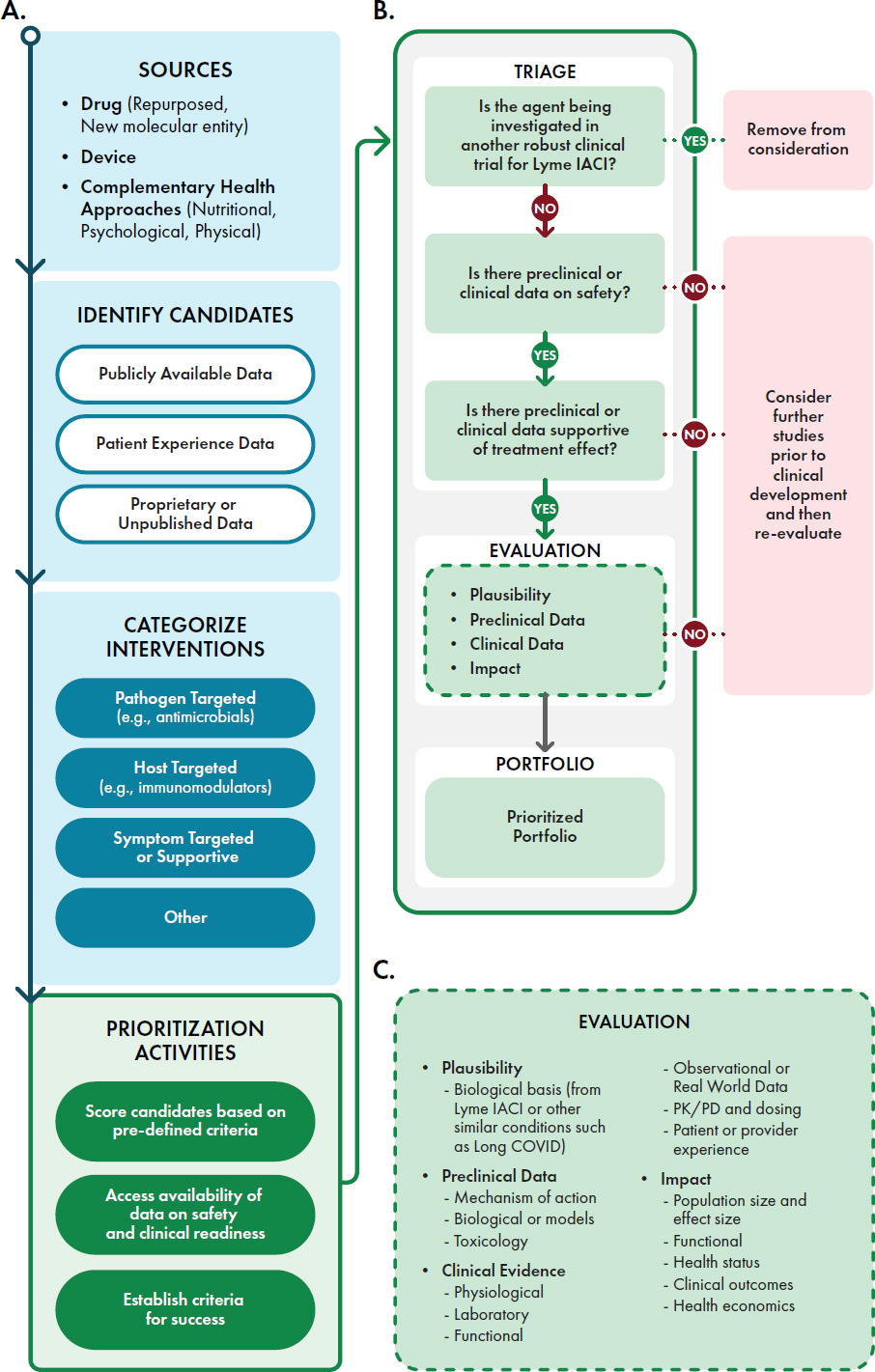
A strategic assessment process is needed to evaluate and prioritize potential treatment candidates for clinical studies. The committee proposes a framework for this prioritization (Figure S-3) that strives to balance a systematic approach with the acknowledgment that different researchers or funders may have different interests that factor into the final decision-making. Potential treatments can be prioritized based on existing evidence, such as preclinical or clinical data demonstrating biological plausibility, safety, and efficacy for a similar symptom from a different condition. Lived experiences from people with Lyme IACI can guide researchers in determining those symptoms that, if addressed, will have the greatest impact on their lives.
Suggesting a shift in focus to clinical studies that address Lyme IACI symptoms does not mean that research on disease mechanisms should be abandoned. To the contrary, continued investigation into biological causes and disease pathways will be important to inform future research. Studies on the etiology and pathogenesis of Lyme IACI and a symptom-driven research agenda for treatments can be explored in a parallel and complementary fashion. To the extent possible, clinical studies evaluating potential treatments for Lyme IACI symptoms would also collect and share data or samples that help elucidate disease mechanisms. For example, clinical data on patient characteristics and disease manifestations or laboratory data, including potential diagnostic or prognostic biomarkers for Lyme IACI, can be collected from randomized, controlled trials for additional analyses.
RECOMMENDATION 1: Research funders should prioritize improving the function and quality of life for people living with Lyme infection-associated chronic illnesses, including the relief of common symptoms, with scientifically supported interventions. To ensure these interventions are supported by robust evidence, clinical studies should be well-designed, randomized trials with appropriate control groups and, whenever possible, include collection of data to help further understanding of disease mechanisms.
BROADENING THE EVIDENCE BASE: COLLABORATION ACROSS SIMILAR CONDITIONS
In this report, the committee explored potential lessons from the published scientific literature by examining systematic reviews on treatments for two chronic conditions that may be triggered by infection and share many symptoms and proposed mechanisms with Lyme IACI: Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). The committee determined that for fatigue, pain, and cognitive dysfunction—the most common symptoms reported by people living with Lyme IACI—there were no interventions from Long COVID or ME/CFS treatment trials that warrant adoption as an immediate priority for Lyme IACI research given the unreplicated, inconsistent, or minor benefits reported in the literature. However, several lessons can be drawn to improve the current Lyme IACI research ecosystem, facilitate a coordinated approach to research between these similar conditions and Lyme IACI, and enable comparison or aggregate analyses of findings between IACI.
Standardize Definitions, Data Collection, and Outcome Measures
Research on ME/CFS and Long COVID has benefited from efforts to standardize clinical definitions and outcome measurements. Without a consensus definition or known diagnostics biomarkers for Lyme IACI, studies have often enrolled participant cohorts that may not be directly comparable to one another. A survey of over 3,000 affected individuals from a large, patient-led data registry reported fatigue, musculoskeletal pain, and cognitive impairment as the most frequent and severe symptoms, which are experienced by 30–62 percent of respondents. Other smaller, prospective observational trials have found similar patterns in symptom prevalence but have not been able to identify unique clinical features for diagnosis. For some individuals, the development of Lyme IACI symptoms may clearly be related to Lyme disease, while for other individuals, symptoms may occur without a clear diagnosis or connection to prior Lyme disease or may be caused by other unknown factors. The certainty of causation between someone’s persistent symptoms and antecedent Lyme disease may depend on the individual’s medical history, such as laboratory testing, previous erythema migrans rash, or recollection of a tick bite. In addition, research suggests that characteristics including biological sex, prior history of trauma, environmental exposures, and individual immunologic differences may be risk factors for developing Lyme IACI, but the degree to which they affect disease risk in all or a subset of individuals is not firmly established. Similarly, multiple mechanistic pathways may lead to persistent symptoms, and the pathways—or combinations of pathways—may differ within the Lyme IACI population.
The heterogeneity in disease symptoms and causal or risk factors for these symptoms suggests the existence of distinct subgroups within Lyme IACI that may inform disease course or treatment outcomes. PTLDS2 is a definition that encompasses some people with persistent symptoms after Lyme disease, but its criteria for a well-documented diagnosis of Lyme disease and significant functional impairment excludes many whose symptom origins are unclear or who experience less severe symptoms but are still deserving of research and treatment. Furthermore, it is difficult to distinguish Lyme IACI from other conditions that share similar clinical presentation due to the emerging but unsettled evidence on diagnostic biomarkers to differentiate among these syndromes. Similar to the approach to defining Long COVID and ME/CFS, developing consensus definitions for Lyme IACI that (1) reflect the established evidence and remaining uncertainties regarding the heterogeneity of this condition, (2) include working definitions for subgroups that may be identified based on clinical or laboratory data, and (3) are reviewed and updated periodically to incorporate new findings are essential for overcoming these challenges and advancing research into this complex condition.
RECOMMENDATION 2: The U.S. Department of Health and Human Services (HHS) should develop consensus research definitions for Lyme infection-associated chronic illnesses (IACI) that address the different strata of the broad range of people living with Lyme IACI.
- HHS should develop a Lyme IACI definition and subgroup definitions that acknowledge the heterogeneity of these illnesses.
- HHS should establish a mechanism to regularly review the Lyme IACI literature and update the consensus research definition and subgroup definitions as new evidence emerges.
- The consensus research definition and subgroup definitions for Lyme IACI should be developed in such a way as to include a broad range of perspectives (e.g., lived experience, clinicians, researchers) and generally align with definitions for other similar conditions and facilitate coordination of research across the diseases.
In addition to helping develop a consensus definition for Lyme IACI, standardizing data collection and analyses could enable data interoperability and harmonize understanding of outcomes between different research
___________________
2 Post-treatment Lyme disease syndrome has been used to describe people who experience persistent and debilitating symptoms for more than 6 months after completing appropriate treatment for a well-documented diagnosis of Lyme disease. This has been defined for research purposes and is not meant as a clinical case definition.
initiatives. Studies on Lyme IACI have not consistently captured the same outcomes or used the same measurement tools for those outcomes, complicating the comparison of results between trials. For example, at least four different tools have been used to measure fatigue in the Lyme IACI literature. One strategy to promote harmonization between studies would be to establish specific, actionable common data elements (CDEs), which would encourage alignment on minimum core datasets to collect in all trials, and also appropriate data-collection tools. As with the prioritization of clinical research candidates, it is important that the development of these research tools, metrics, and data standards include input from those living with Lyme IACI, including those currently underrepresented in research, such as children, so that research outcomes address the experience and goals of those affected by the condition.
RECOMMENDATION 3: The National Institutes of Health (NIH), in coordination with the Centers for Disease Control and Prevention (CDC), should define a set of standard research tools and metrics to advance research and development of new treatments for Lyme infection-associated chronic illnesses (IACI). These include common data elements (CDEs), sensitive outcome measures, and terminologies that reflect the lived experience of people with Lyme IACI.
- NIH and CDC should assess, with participation of all interested parties, whether existing patient-reported outcome measurement tools reliably and accurately capture the priority outcomes for people with Lyme IACI, or if new tools and measures are needed. This should include determining if there are groups (e.g., children) for which existing reporting tools do not capture necessary Lyme IACI constructs.
- NIH and CDC should evaluate existing CDEs from myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID for components that can be incorporated into Lyme IACI CDEs to enable knowledge sharing among IACI, including through comparative studies of multiple disease areas. This could include adopting a core set of clinical characteristics for study participants, study methodologies, and symptom reporting questionnaires that mirrors those already being used in ME/CFS or Long COVID research.
Expand Data Collection
The heterogeneity of Lyme IACI symptoms and potential pathophysiologies suggests that a large collection of useful data (e.g., epidemiologic, clinical, or laboratory findings from well-characterized treatment trials or prospective, longitudinal cohorts) is needed to untangle complex disease pathways or distinguish meaningful biological signals for disease prognosis and treatment response from background noise.
Sources for broadened data sampling that also improve the patient-centeredness of research endeavors would include patient registries that collect self-reported data directly from patients and biobanks that catalog biological material along with associated health information. Both patient registries and biobanks are underused in Lyme IACI research. Of 42 Lyme IACI research articles that mentioned the use of biological samples, just three reported obtaining those samples from a biobank. While data from patient registries and biobanks cannot substitute for randomized trials, the adoption of infrastructure and best practices to support the quality, standardization, and sustainability of these data streams would enable them to better complement clinical research. A coordinated research strategy between Lyme IACI and other similar conditions can also expand the pool of useful data across diseases with common symptoms, though careful data characterization and stratification will be critical to identify commonalities and differences among conditions. Artificial intelligence (AI) tools may be used to overcome challenges in analyzing the large volumes of data. For example, AI applications could be developed to analyze unstructured data from a patient registry and generate testable hypotheses on mechanistic pathways for Lyme IACI, or to predict subpopulations based on various sources of clinical and laboratory data.
RECOMMENDATION 4: To enhance impact in research, funders and managers of biobanks and patient registries for Lyme infection-associated chronic illnesses (IACI) should adopt the following practices that optimize the sustainability of these resources and the accessibility, quality, and utility of their samples.
- Biobanks should promote awareness, coordination, governance, accessibility, sustainability, and standardization of data and samples from participants with Lyme IACI and those serving as appropriate control groups, including healthy controls and participants with other similar conditions or symptoms.
- Biobanks and patient registries should refine and make public the data domains they capture to increase accessibility and spur collaboration. In sample and data collection, the samples should be characterized in a manner that promotes data quality
- and confidence in their use. Biological collections associated with individual research studies should follow a standardized set of basic data and metadata domains to facilitate use across studies.
- Biobanks and patient registries should develop and communicate protocols that describe the intended use of collected samples and data for participants who contribute their data and samples.
Collaborate and Coordinate
Lyme IACI research currently does not have a coordination mechanism. This inhibits both collaboration and the effective sharing of information and resources across research sites. The expansion of trial networks will be important to developing a concerted strategy within Lyme IACI research and to connecting with research advances in other similar conditions, but supporting such an expansion will require significant infrastructure. An effective model that has been applied to ME/CFS and Long COVID research is a data-coordinating center that promotes collaboration, improves interoperability, and reduces administrative burden on investigators in complex and interdisciplinary research.
RECOMMENDATION 5: Research funders should support the development and sustainment of a Lyme infection-associated chronic illnesses (IACI) research data-coordinating center that facilitates resource and knowledge sharing across programs conducting Lyme IACI clinical research and incorporates input from people living with Lyme IACI.
- To further the visibility of biobank resources, the research data coordinating center should collaborate with biobanks on the development of a central repository that catalogues the location and characteristics of available samples and data.
There is an unrealized opportunity for researchers, clinicians, and people living with Lyme IACI and other conditions with similar clinical features to share knowledge, best practices, and other learnings to understand and address what may be common mechanistic pathways or effective treatments across the different syndromes. Pooling research efforts to examine biological processes that may be at the root of these similar conditions can streamline the discovery, validation, and translation of findings to maximize impact from the available funding and resources. For example, this coordinated approach could identify new treatments that are effective in treating symptoms shared by more than one condition (e.g., an anti-inflammatory
drug that works for fatigue in ME/CFS and Lyme IACI) while reducing the inefficiencies of conducting individual, sequential studies for each disease. These studies need to be designed carefully to ensure scientific rigor and patient-centeredness, with an appropriate stratification of the study participants for meaningful and robust analyses of the specific pathogen trigger, additional risk factors, or host characteristics.
RECOMMENDATION 6: The Department of Health and Human Services (HHS) should develop an integrated strategic plan for infection-associated chronic illnesses (IACI) research that facilitates collaboration across the different disease research efforts. The strategic plan should improve the understanding of commonalities among IACI and identify and advance interventions to address specific conditions, including Lyme IACI. The strategic plan should balance the need for clinical research on treatments, basic and clinical research on disease mechanisms, and incorporation of real-world evidence (RWE).
- The strategic plan should prioritize the development and support of substantive efforts to improve the treatment and management of IACI symptoms.
- The strategic plan should include continued investment in large-scale, prospective, multicenter observational studies designed to generate evidence on the mechanistic similarities and differences between Lyme IACI and other IACI, including clinical and laboratory characteristics.
- To complement prospective studies, the strategic plan should address the opportunity to use RWE, including information based on patient registry data and findings from observational studies.
TIME FOR ACTION
As this report lays out, the current understanding of the causes and mechanisms of Lyme IACI and how to best treat the disease state remain unclear. Yet there is urgency to proceed with research to identify and develop safe and effective treatments that can restore functionality and quality of life despite these uncertainties, as many people continue seeking relief from debilitating symptoms of Lyme IACI in the vacuum of evidence-based treatments for this condition. Research capable of delivering effective Lyme IACI treatments can be conducted now. However, there needs to be a paradigm shift in how researchers, clinicians, and funders navigate the uncertainties and complexities within this field: specifically, researchers should prioritize the investigation of treatments to address the symptoms of
Lyme IACI, while investigations of the disease mechanism occur in parallel with or as a complement to the treatment-oriented efforts. The Lyme IACI field should take advantage of this paradigm shift to enhance standardization and coordination among its dedicated researchers, clinicians, people living with Lyme IACI, and funders for these efforts. Furthermore, there is a critical opportunity to advance Lyme IACI research through enhanced collaboration with related disease areas such as Long COVID and ME/CFS. The committee’s six recommendations chart the path to concerted action on research that will benefit the individuals living with Lyme IACI.















