Challenges in Supply, Market Competition, and Regulation of Infant Formula in the United States (2024)
Chapter: Summary
Summary1
In late 2021 and early 2022, the U.S. Food and Drug Administration (FDA) received information about four cases of infant illness or death after consuming powdered infant formula. Abbott Nutrition issued a voluntary recall of certain infant formula products and temporarily ceased production at its largest site. This caused a widespread shortage, unprecedented in scope and duration, that created hardships for families who used infant formula.
In the 2023 Consolidated Appropriations Act, Congress provided funding for FDA to contract with the National Academies of Sciences, Engineering, and Medicine (the National Academies) to examine and report on the challenges in supply, market competition, and regulation of infant formula and other related topics. The committee’s statement of task is shown below (Box S-1). FDA also provided additional topics for the committee to consider (see Chapter 1). The National Academies convened a committee with expertise spanning public health safety and policy; public health nutrition and policy; infant health and nutrition; equity in access to formula supply; business and economics, including supply chain dynamics; and regulatory standards and oversight. The committee interpreted the task as a request to: (1) explain the vulnerabilities that were exposed during the shortage; (2) describe the extent to which actions taken by relevant stakeholders have addressed these
___________________
1 This summary does not include references. Citations for findings presented in the summary appear in the subsequent chapters of the report.
BOX S-1
Statement of Task
An ad hoc committee of the National Academies of Sciences, Engineering, and Medicine will examine and report on challenges in supply, market competition, and regulation of infant formula in the United States. Specifically, the committee will assess and evaluate:
- Characteristics of the U.S. infant formula market, including number, market share, and other properties;
- Challenges in supply, or market competition with respect to such formula;
- Differences in infant formula marketed in the United States and infant formula marketed in the European Union, including with respect to nutritional content and applicable labeling and other regulatory requirements; and
- Other related information.
The committee will produce a report of its findings, conclusions, and recommendations, including recommendations for infant formula manufacturers, on measures to address supply and market competition in the United States as described in H.R. 2617 of the FY2023 Omnibus Bill.
vulnerabilities; (3) identify the gaps that remain to be addressed; and (4) recommend how to address them.
THE COMMITTEE’S APPROACH
The committee was motivated by the importance of early life nutrition in child health, as infant formula is the primary or supplementary source of nutrition for many infants. The committee considered the supply chain from the production of ingredients through the acquisition of infant formula by families, as well as barriers to accessing infant formula in certain populations before, during, and after the 2022 infant formula shortage. The committee also considered the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) as an important stakeholder as it has been estimated that infants participating in WIC consume more than half of the infant formula in the United States.
The committee held closed and public meetings to understand the challenges in supply, market concentration, and regulation of infant formula; it also gathered written information from many stakeholders and reviewed scientific literature, federal documents, and relevant websites.
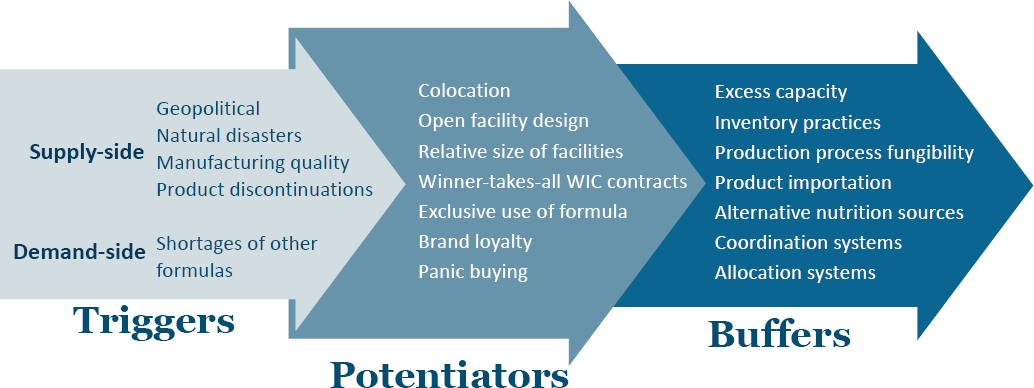
The committee also developed a detailed timeline, which covered the actions of many federal departments, as well as supply chain diagrams for infant formula during and after the 2022 shortage. The committee considered a vulnerability framework originally developed for prescription drug supply chains to assess the vulnerability of the infant formula supply chain (see Figure S-1). The framework differentiates between triggers (events or actions that disrupt the supply of a key ingredient or infant formula); potentiators (factors that exacerbate the shock to the system); and buffers (policies or practices that mitigate the effect of a supply disruption).
BACKGROUND
The infant formula market for healthy, term infants in the United States contains products that differ in their source of proteins, carbohydrates, and fats; their degree of protein hydrolysis; and their inclusion of optional components that are not part of the essential composition. These infant formulas are called non-exempt in this report because they are not exempt from FDA regulation nutrient requirements. Infant formulas that are exempt from some FDA regulation of nutrient requirements are for infants with specific medical needs. Before infants are 6 months old, infant formula alone is designed to meet their nutritional needs. They are not needed for fully breastfed infants and complement breast milk intake for partially breastfed infants. The committee examined the infant formula supply chain and government framework before, during, and after the 2022 infant formula shortage.
Before the 2022 Shortage
The production and marketing of infant formula in the United States is subject to a complex regulatory environment that encompasses product ingredients, nutritional quality, manufacturing safety, product packaging and labeling, and importation. Numerous federal departments are involved in assuring the quality and safety of this critical product: FDA, the U.S. Department of Agriculture (USDA), the Centers for Medicare & Medicaid, and the Centers for Disease Control and Prevention (CDC). To market infant formula in the United States, a manufacturer, whether production is domestic or foreign, must be registered with FDA and, 90 days prior to introducing their infant formula into the U.S. market, the manufacturer must go through a notification process. WIC provides infant formula to its low-income participants, which adds another layer of regulatory complexity. Although the U.S. regulatory framework for infant
formula is consistent with the international Codex standard, there are differences between U.S. regulations and those of other countries.
The infant formula supply chain consists of upstream, manufacturing, downstream, and consumers (see Figure S-2). The upstream supply chain includes ingredients and packaging materials (i.e., containers and labels) used in the production of infant formula. Infant formulas may contain imported components (e.g., nutrient premixes2). Most U.S. manufacturers generally make the product sold under their brands, but one is a contract manufacturer specializing in making brands for smaller companies as well as private-label brands. All FDA-registered manufacturing facilities (domestic or foreign) are subject to FDA inspection.
Although infant formula is available as liquids (concentrated or ready-to-feed), most infant formula sold in the United States as of 2024 is in powdered form. Each form of infant formula requires different manufacturing processes and materials. Moreover, lines to produce powdered infant formula cannot be switched to make liquids during a shortage. Unlike the liquid form, powdered infant formula is not sterile and it is difficult to determine if it is contaminated. One contaminant of concern is Cronobacter sakazakii, which can cause serious and potentially life-threatening infections, especially in young infants. Cronobacter sakazakii has been found in homes, manufacturing plants, and other environments.
The supply side of the infant formula market is highly concentrated at multiple levels, including production capacity and sales. Production capacity refers to the concentration of infant formula production across and within a manufacturer’s facilities. Both the number of production sites and sizes of these facilities vary by manufacturer, and some sites may be the sole producers of certain products. In the United States in 2022, the top two infant formula suppliers make up 66 percent of the market, which is less than it was in the 1980s. The brand level of U.S. concentration is comparable to that of other developed countries.
The downstream supply chain includes distribution from the manufacturer, either directly or via a distributor, to the vendor. Distribution of infant formula to hospitals may involve a group purchasing organization. Most infant formula is purchased by hospitals and caregivers (either on their own or redeemed using program benefits by WIC participants).
Many factors influence why and how infants receive infant formula. U.S. caregivers are inundated with marketing messaging about infant formula, which affects their purchasing decisions. Health care providers are one of caregivers’ first resources for guidance on feeding infant formula, especially about switching formulas when caregivers perceive tolerance
___________________
2 “Nutrient premix means a combination of ingredients containing two or more nutrients received from a supplier or prepared by an infant formula manufacturer” (21 CFR § 106.3)
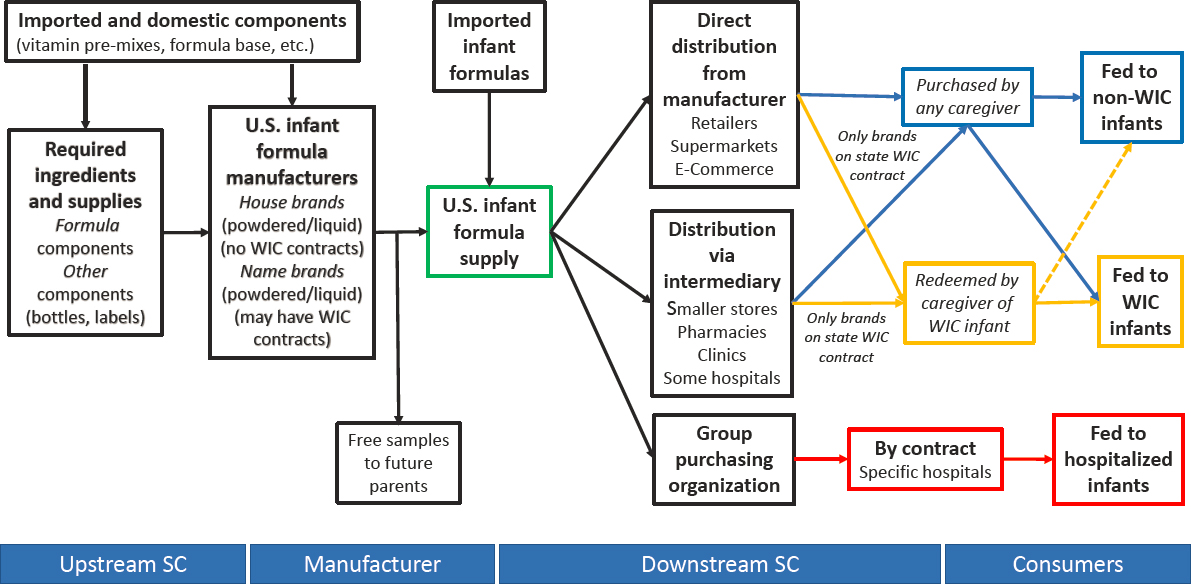
NOTE: SC = supply chain; WIC = Special Supplemental Nutrition Program for Women, Infants, and Children.
issues or when a desired brand or product is unavailable. Even though many infant formula products resemble one another, without education on variations in infant formula ingredients and selection, it is difficult for consumers and health care providers to identify or select substitute products when needed.
WIC
WIC provides supplemental nutrition support for income-eligible, pregnant, and postpartum participants, and infants and children under age 5, which includes providing infant formula. About half of the infant formula purchased each year is provided to WIC participants, and they redeem their infant formula using an electronic benefit card only at WIC-authorized vendors. The infant formula brand issued by state WIC agencies is determined by a competitive bidding process. It has resulted in significant price discounts for WIC, in the form of rebates, enabling WIC to serve more participants. The winning bidder also has marketing advantages in the non-WIC market (e.g., shelf space, use by hospitals).
2022 Infant Formula Shortage
When Cronobacter sakazakii was found at Abbott’s Sturgis, Michigan plant, FDA issued warnings and recommended that Abbott voluntarily recall powdered infant formulas. Abbott implemented a recall and eventually paused production of infant formula at this facility, which created a shortage that rapidly became widespread and affected families of all income levels that used infant formula. This affected not only non-exempt infant formula but also exempt formulas for individuals with medical or dietary conditions that have limited to no other options.
For many families, the shortage required switching infant formula brands, but few resources were available to guide caregivers through appropriate substitution strategies. WIC participants experienced additional, unique challenges related to their inability to find and redeem permitted brands of infant formula. Meanwhile, WIC-eligible, nonparticipating individuals were more likely to use suboptimal coping strategies. It is noteworthy that both panic buying and inadvertent stockpiling occurred, exacerbating the shortage.
During the shortage, some hospitals provided limited quantities of infant formula to special-needs patients after discharge, but patients generally procured infant formula from sources outside the hospital after discharge. Health care providers were concerned their patients would not be able to access safe, medically indicated infant formula after hospital discharge. Larger hospital centers were more able to navigate the crisis
by substituting infant formulas, having greater support for purchasing departments and designated manufacturer sales representatives, and better inventory control, which mitigated some of the negative effects on infants and their families.
Distribution of the available infant formula was especially problematic for WIC (see Figure S-3). The states where Abbott held the WIC contract were affected more rapidly and severely by the shortage. Abbott voluntarily agreed to pay the rebate to state WIC agencies for other brands of infant formula. State WIC agencies lacked information about the distribution of infant formula, making it difficult for them to redirect the available product where needed. As a result, redemption rates for infant formula dropped sharply in some locations. Meanwhile, there was a striking increase in the proportion of WIC participants who chose to breastfeed their newborns.
Response to the 2022 Shortage
Multiple federal agencies, the White House, and Congress took actions both during and after the 2022 infant formula shortage to mitigate its effect on U.S. families and prevent future shortages (see Figure S-4, detailed information in Appendix C). These actions included legislative and regulatory policies focused on education, enforcement discretion (i.e., not enforcing a regulatory requirement if certain criteria are met by infant formula manufacturers), and new requirements related to disruptions, recalls, shortages, and reporting. Federal agencies continue to implement laws that were enacted in response to the shortage and take actions to prevent future infant formula shortages.
While sales in the U.S. market are highly concentrated at the national and state levels, the concentration in production is most relevant to the likelihood of a shortage and the ability to buffer the effects of a shortage.
A few months after the closure of Abbott’s plant in Sturgis, Michigan, FDA issued guidance explaining how it would exercise enforcement discretion for products that were not currently registered in the United States to help increase the supply of infant formula. Shortly after, Congress enacted the Access to Baby Formula Act of 2022 (ABFA) to create standing authority to waive WIC rules during supply disruptions, allow USDA to work with the U.S. Department of Health and Human Services to facilitate communication and coordination regarding supply chain disruptions, and require each state WIC agency infant formula contract to include language on remedies in the event of a recall.
Congress also temporarily suspended tariffs on infant formula and its ingredients. The White House coordinated the response and created its “Fly Formula” operation to bring international formula into the United
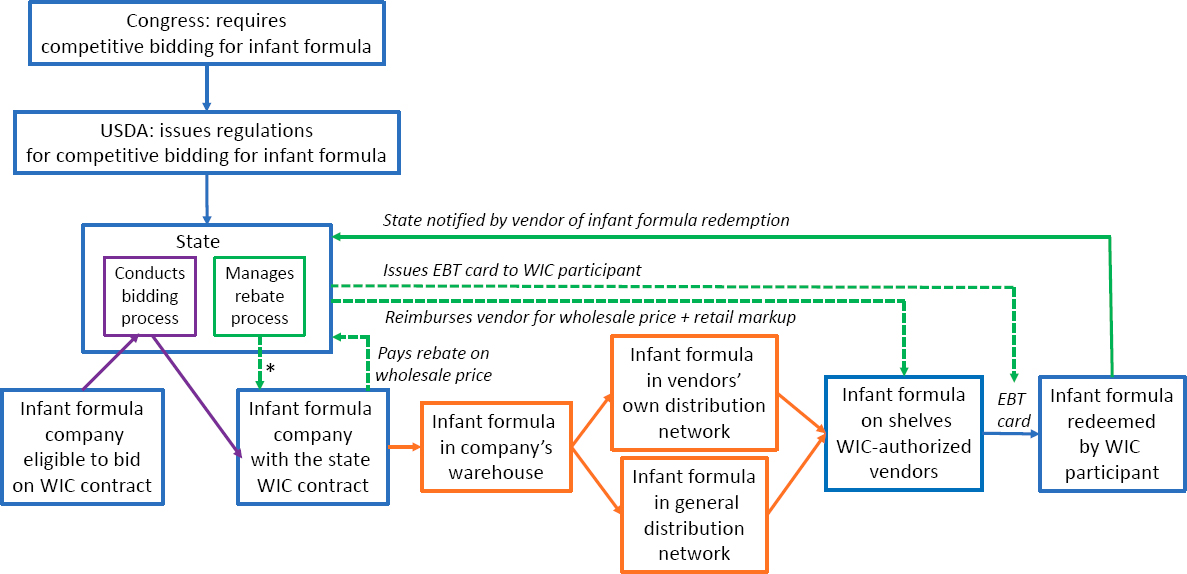
NOTES: *WIC invoices the company for the redeemed infant formula. WIC = Special Supplemental Nutrition Program for Women, Infants, and Children; EBT = electronic benefit transfer; USDA = U.S. Department of Agriculture.
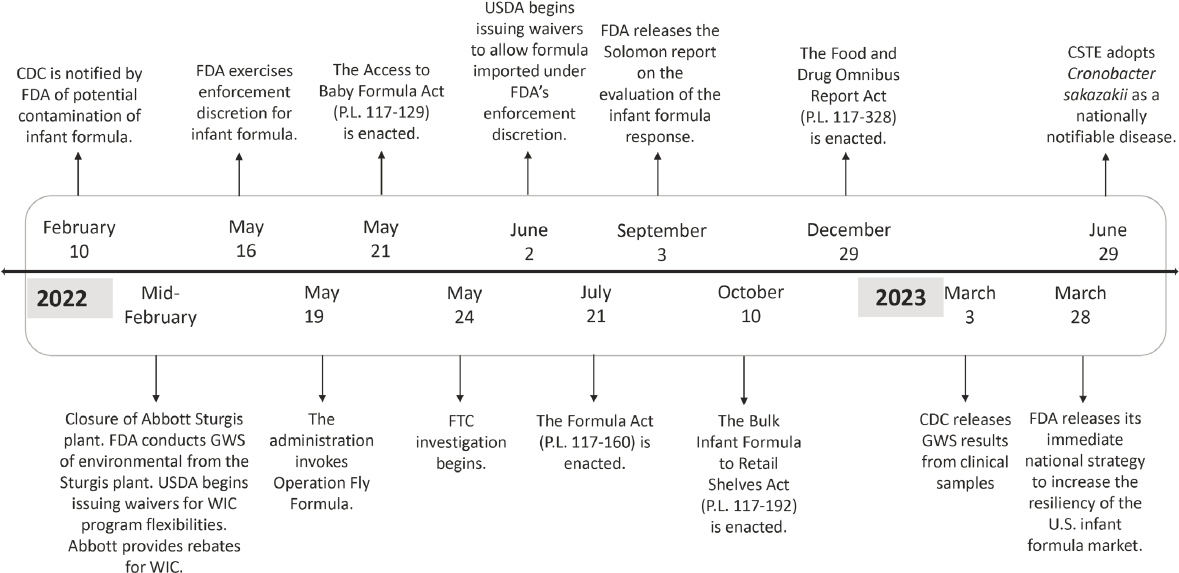
NOTES: CDC = Centers for Disease Control and Prevention; FDA = U.S. Food and Drug Administration; USDA = U.S. Depart ment of Agriculture; CSTE = Council of State and Territorial Epidemiologists; GWS = genome-wide sequencing; WIC = Specia Supplemental Nutrition Program for Women, Infants, and Children; FTC = Federal Trade Commission.
States. Also, although U.S.-based manufacturers made frequent and substantial changes to production, their ability to increase supply was limited by production capacity and availability of inputs.
Late in 2022, the Food and Drug Omnibus Reform Act of 2022 (FDORA) was enacted. It requires FDA to review the list of nutrients in infant formula and consider revising the nutrients (or their levels) in accordance with its findings, and consider new data related to international formula standards. FDORA had additional provisions related to recalls, inspections, national strategies, personal importation, and labeling. Notably, FDORA established a new category of foods, namely “critical foods,” which includes infant formula and medical foods. FDORA also establishes requirements for infant formula manufacturers to notify FDA of interruptions or discontinuances that can cause a meaningful disruption, as defined in FDORA, and a requirement for each manufacturer to develop a redundancy risk management plan (RRMP).
Vulnerabilities Identified
After assessing the infant formula landscape before, during, and in response to the 2022 infant formula shortage, the committee found vulnerabilities in the following five areas:
- Risk management planning: Risk management planning and the implementation of such plans by the federal government and infant formula companies are key elements of a preparedness strategy for potential supply disruptions. Lack of understanding of potential risks to the supply of infant formula and the investments needed to prevent or minimize shortages has the potential to contribute to future shortages.
- Production concentration: Infant formula sales are concentrated at the national and state levels, but it is the concentration of production that creates a greater vulnerability to supply disruptions. Production disruptions at a single facility, which is likely because some are outdated, can create significant supply disruptions relative to the size of the market.
- Support for speedy supply recovery: The restoration of production was slowed by certain aspects of FDA’s authority for oversight of manufacturing as well as regulatory requirements that inhibited rapid importation of infant formula.
- Government’s management of adverse consumer impact: Adverse effects were heightened by inadequate communication with the public, unique constraints faced by WIC program participants, and a lack of coordination between government and industry.
- Protection, promotion, and support of breastfeeding: Structural barriers manifested through lack of access to qualified breastfeeding protection and support contributes to the dependence on an adequate supply of infant formula.
THE COMMITTEE’S CONCLUSIONS AND RECOMMENDATIONS
The committee describes its recommendations as they relate to the vulnerabilities identified above.
Risk Management Planning
Proper risk management planning for potential supply disruptions needs to be conducted by the federal government and infant formula companies at the sector, firm, facility, and product levels. FDORA requires that manufacturers develop RRMPs. The 2022 infant formula shortage highlighted an inadequacy of risk management preparedness and response on multiple levels.
The committee concluded that sector-wide resiliency planning is needed to reduce the likelihood that a supply chain disruption is triggered and to buffer a disruption. FDORA’s requirement that all infant formula manufacturers develop RRMPs is a critical first step in preparing for supply disruptions. The implementation of such plans is essential. Evidence-based regulatory standards that reflect the risk management profile of the facility and the individual product’s supply chain are needed to support the development of adequate plans to prevent or contain disruptions. The federal government must ensure that WIC contract holders develop and implement RRMPs that meet FDA’s expectations because WIC provides a high proportion of the infant formula consumed in the United States.
Recommendation 1: The U.S. Department of Health and Human Services and the Food and Drug Administration, including the Office of Critical Foods, should conduct sector-wide risk management planning.
Recommendation 2: The Food and Drug Administration (FDA) should develop evidence-based redundancy risk management plan (RRMP) regulatory standards, a process for reviewing RRMPs, and a mechanism for monitoring implementation of the RRMPs. The committee proposes two options to accomplish this recommendation:
- Legislative option: Congress should amend the Infant Formula Act to require all manufacturers to submit
- Administrative option: FDA should use its authority under the Food Safety Modernization Act to promulgate regulations that require RRMPs for critical food facilities as part of their required food safety plans and establish evidence-based standards and a review process for RRMPs.
RRMPs for each product, while recognizing capacity constraints, and direct FDA to promulgate regulations specifying evidence-based regulatory requirements for RRMPs and an RRMP review process to provide feedback on compliance with the evidence-based standards.
Although FDORA requires RRMPs, the committee notes that it is important to have evidence-based regulatory requirements, a review process, and feedback on compliance. The legislative option is preferred with Congress providing FDA broader authority to promulgate regulations specifically addressing RRMPs. In the absence of legislation, at a minimum, FDA could use its authorities in the Food Safety Modernization Act to require RRMPs as part of facilities’ food safety plans.
Recommendation 3: The U.S. Department of Agriculture should promulgate regulations requiring an infant formula manufacturer to meet the Food and Drug Administration’s expectations with regard to evidence-based redundancy risk management plan standards to be considered a “responsive” bidder under 7 C.F.R. § 246.16a(c)(5).
Market Concentration
Any disruption in concentrated manufacturing facilities can affect the supply of infant formula to the market, as happened in 2022. Understanding and addressing concentration at multiple levels can help improve the resiliency of the infant formula market in the United States.
The committee concluded that the relevant concentration measure for supply chain reliability is the concentration of production capacity, not the number of companies marketing products or the sales concentration of those companies. Infant formula production capacity in the United States is likely concentrated, which enhances the potential of a shortage, requiring larger buffering strategies.
Recommendation 4: Congress should provide suitable incentives (e.g., offering tax credits, accelerated depreciation) to
encourage all Food and Drug Administration (FDA)-registered infant formula manufacturers that have met FDA expectations with regard to evidence-based redundancy risk management plan (RRMP) standards to implement the RRMP and modernize manufacturing plants and equipment located in the United States. This modernization should include measures to prevent the spread of possible contaminants from one area of a facility to another, thereby ensuring product safety and minimizing the size and scope of a plant shutdown following the identification of contamination.
The committee concluded that WIC’s competitive bidding process is not the driver of industry concentration at the national level. Eliminating WIC’s competitive bidding process for infant formula would likely lead to higher wholesale prices and, ultimately, higher retail prices for infant formula for all consumers, higher federal WIC costs, and increased incentives for manufacturers to market infant formula to WIC participants. Currently, the best way to protect consumers from the effects of a disruption to the dominant state brand is through building on the WIC contract remedies required under the ABFA. The committee concluded that the specific remedies that USDA now requires WIC infant formula contracts to include would not help during shortages precipitated by factors other than a recall.
Recommendation 5: The U.S. Department of Agriculture (USDA) should extend some of the required infant formula contract remedies to emergency periods or supply chain disruptions precipitated by factors other than a recall and facilitate infant formula distribution to vendors authorized by Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) during disruptions to protect consumers from the effects of a disruption to the WIC contract-holder’s supply.
-
USDA should revise 7 C.F.R. § 246.16a(n) to require that during all emergency periods and supply chain disruptions,
- contracts permit the issuance, without medical documentation, of infant formula in container sizes, physical forms, and brands that are not usually permitted; and
- the contract holder provides the state WIC agency with action plans that include supply data.
- USDA should provide states with template contract language and guidance to facilitate implementation of all the contract remedies in 7 C.F.R. § 246.16a(n).
-
USDA should revise 7 C.F.R. § 246.16a(n) to require WIC infant formula contracts to specify that the contract holder will
- distribute infant formula based proportionally on past allocations during an emergency period or supply chain disruption; and
- modify its distribution contracts to require infant formula to be distributed based proportionally on past allocations during an emergency period or supply chain disruption.
- USDA should update 7 C.F.R. § 246.12(g)(9) and § 246.12(h) (3)(xvi) to require WIC-authorized vendors to provide a list of infant formula wholesalers and distributors that they use, upon authorization and upon subsequent request by USDA or the state WIC agency.
Speedy Supply Recovery
The ability to respond quickly to a shortage is important regardless of the cause. Although actions were taken in response to the 2022 infant formula shortage, further preparation could help increase the speed of response to future shortages.
The committee concluded that a lack of advanced notice about an interruption or discontinuation that is likely to lead to a meaningful disruption could result in a preventable shortage when such interruption or discontinuation is a business decision that a manufacturer knows about in advance.
Recommendation 6: Congress should amend the Federal Food, Drug, and Cosmetic Act to require manufacturers of critical foods to give sufficient advanced notice to the Food and Drug Administration when they decide to discontinue a critical food that is likely to lead to a meaningful disruption prior to removing the product from the market temporarily or permanently.
The committee concluded that authority for remote access to inspection records can strengthen efforts for both ongoing oversight of critical foods and managing the safety and quality of these foods during a crisis.
Recommendation 7: Congress should grant the Food and Drug Administration the authority for remote access of records for critical foods.
The committee concluded that understanding barriers to entry (e.g., tariffs and regulatory requirements) during non-shortage time will provide information on necessary actions during any shortage.
Recommendation 8: The Food and Drug Administration (FDA) should collect information on nutrient and labeling requirements used by other countries and use this information to develop a database to be updated every 4 years, consistent with the Food and Drug Omnibus Reform Act of 2022, of the requirements in other countries to facilitate the use of enforcement discretion in case of a shortage. In addition, the information in the database can be used by FDA to evaluate evidence to determine if an update is needed to U.S. regulations.
Recommendation 9: Congress should establish a trigger rule to suspend tariffs and tariff-rate quotas automatically on imported infant formula and imported inputs used in the domestic production of infant formula in the event of a meaningful disruption to the market, as defined by the U.S. Department of Health and Human Services.
The committee concluded that government officials tasked with monitoring supply conditions did not seem immediately aware that distributors are important points of contact for information about the flow of product. The government was also not able to obtain the information needed to identify where infant formula (i.e., now classified as a critical food in FDORA) was in the supply chain, which hindered its ability to adequately coordinate the whole-of-government response.
Recommendation 10: The U.S. Department of Health and Human Services (HHS), including the HHS Supply Chain Coordinator and the Food and Drug Administration, should work with the appropriate wholesalers and distributors of infant formula to develop risk management and disaster plans to prepare for a shortage of critical foods.
Management of Adverse Consumer Effects
During the shortage, caregivers generally lacked an understanding of how to handle and prepare powdered infant formula safely. Although
WIC was critical in allowing the participants to obtain infant formula during the shortage, WIC participants were more constrained than other customers in obtaining infant formula. Infants with metabolic conditions, who require exempt infant formulas, have a particularly limited set of feeding options because the use of other formulas or even human milk can lead to severe health problems.
The committee concluded that consumers and health care providers struggled more than usual to identify and select appropriate substitute products during the shortage. All of the government guidelines about the preparation of infant formula should continue to be identical to ensure that consistent messages are disseminated to health care providers to disseminate to patients, and to manufacturers to share with consumers.
Recommendation 11: The Food and Drug Administration and the Centers for Disease Control and Prevention should work with infant nutrition experts and infant formula manufacturers to jointly develop guidance on how caregivers can substitute infant formula (based on ingredients) and guidance for providers and caregivers on how to feed their infants when no breast milk or safe infant formula is available, to address ongoing challenges, and to prepare for any future recall or supply disruption.
The committee concludes that the requirement in FDORA to provide information on appropriate substitutes for certain products (inborn errors of metabolism or other serious health conditions, such as prematurity) is important, but it does not address the need to develop a list of appropriate substitutes for non-exempt and hypoallergenic formulas.
Recommendation 12: The Food and Drug Administration should maintain a public list of all infant formulas currently marketed and registered in the United States, indexing formula name, whether the formula is exempt or non-exempt, and the registered list of ingredients that appear on the label.
The committee concluded that updating USDA’s 2021 Guide to Coordinating Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) Services When Regular Operations Are Disrupted (2021 USDA Guide) and strengthening requirements regarding state plans for alternative operating procedures are essential to buffer future shortages.
Recommendation 13: The U.S. Department of Agriculture (USDA) should update the 2021 USDA Guide and requirements regarding state alternative operating procedure plans in 7 C.F.R.
§ 246.4(a)(30) to include a design of a governance structure for crisis response that articulates all stakeholders that should be involved, how they should work together, what is expected of them, and how the overall response should be coordinated at the state level. USDA should also develop a dashboard to track critical data on the supply and shortages of infant formula at the state or national level.
The committee concluded that, because of the more restrictive set of options faced by users of most exempt formulas, special considerations are needed to ensure a steady supply of those formulas, including protecting the supply against demand spillovers.
Recommendation 14: The Centers for Medicare & Medicaid Services should issue a rule requiring hospitals that accept Medicaid to have a plan for responding to a meaningful disruption of nutrition support for hospitalized infants.
Breastfeeding Protection, Promotion, and Support
For many families, formula feeding is desired. For other families, breastfeeding is desired, although it may or may not always be possible. Families of breastfed infants were protected from the challenges created by the shortage of infant formula. However, for some families, breastfeeding may not be possible because medical problems or structural barriers impede their choice to breastfeed, and as a result, although the proportion of families who choose to breastfeed has increased in recent years, it is still lower in the United States than in other similar countries, and lower still among families served by WIC.
The committee concluded that breastfeeding reduced the proportion of families affected or severely affected by the infant formula shortage and that attention to breastfeeding promotion, initiation, and support remains essential for infant health.
Areas for Additional Research
During the committee’s deliberations and development of its conclusions and recommendations, a key message arose that further research and data are needed. The areas that should be explored further are related to
- the effect of tariffs on the infant formula market,
- how to aid the expansion of infant formula companies that lack some marginal capacity to bid on WIC infant formula contracts,
- how to ensure the WIC program has sufficient funding during non-recall shortages,
- development of sufficient buffering capacity for medically necessary products, and
- analysis of the notification process for new3 infant formulas (referred to in this report as the new infant formula notification process).
The committee’s full discussion of research and data needs are presented in Chapter 6.
GOING FORWARD: FINAL CONSIDERATIONS
The committee worked with three overarching perspectives, which include what and how to feed infants, how supply chains can ensure reliable and equitable access to safe infant formula for all users, including for WIC, and preparation for future infant formula supply shocks. Infant formula, the focus of the committee’s work, is an essential food for infants whose parents choose to or must use this product. The committee acknowledges that the use of breast milk and infant formula interact with one another—within families and in society at large. Families may use breast milk, infant formula, or both—simultaneously or sequentially. In the context of the shortage, the importance of breastfeeding was a mitigating factor for families. The cost of infant formula alone can make it unaffordable for some low-income families. As a result, WIC plays an essential role in providing infant formula to infants in these families at no charge. The committee was attentive to policy recommendations that preserve the ability of WIC to serve all eligible families that seek to participate.
In developing its conclusions and recommendations, the committee was fully aware that building resiliency and having reliable supply chains for infant formula requires a financial investment and a deliberate approach to who will bear those costs. Throughout its deliberations, the committee paid close attention to the legislation enacted in response to the shortage, the published reports commissioned by FDA and the Federal Trade Commission, guidance or regulations issued by federal agencies, and ongoing work on related topics. The committee acknowledged the importance of these actions and identified where additional steps are necessary.
The infant formula shortage of 2022 revealed the inadequacy of risk management planning by both the infant formula industry and the fed-
___________________
3 The notification process is required for infant formulas that are new to the market and for certain changes made to existing infant formula (see Chapter 3).
eral government, and the consequence was an exceptionally challenging situation for both families and WIC. While the 2022 infant formula shortage was triggered by a specific cause, Cronobacter sakazakii contamination is not the only potential risk to our infant formula supply chain. Consequently, the report recommends comprehensive, cross-departmental study of risks, which could result in the need to request additional authorities and resources through congressional action. The insights gained from this analysis, when implemented, should provide additional protection for critical foods.
The consequences of the infant formula shortage of 2022 were felt by nearly all families feeding infant formula and affected most companies in the industry. This distress was most acute among low-income families and those with infants requiring exempt infant formula. The committee’s analyses revealed ways such shortages could be prevented or mitigated. These analyses also revealed new concerns related to the vulnerability of the whole infant formula supply chain, which is subject to geopolitical and “force of nature” threats that could result in more widespread shortages of longer duration. The committee’s conclusions and recommendations are intended to ensure that the United States is better positioned to respond to any future shortage.



















