Ensuring Safe Foods and Medical Products Through Stronger Regulatory Systems Abroad (2012)
Chapter: Appendix C: Food and Medical Product Regulatory Systems of South Africa, Brazil, India, and China
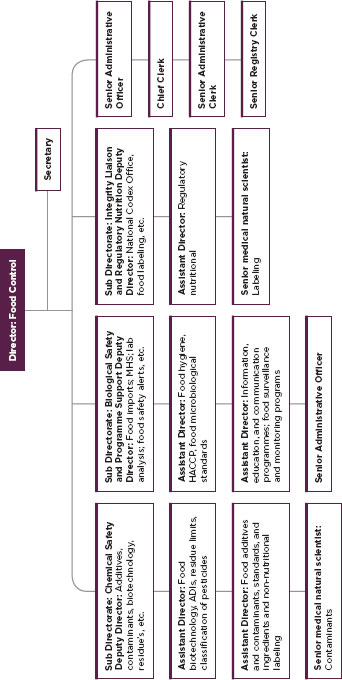
FIGURE C-1
Organization of the South African Food Control Directorate.
SOURCE: South African Food Control Directorate.
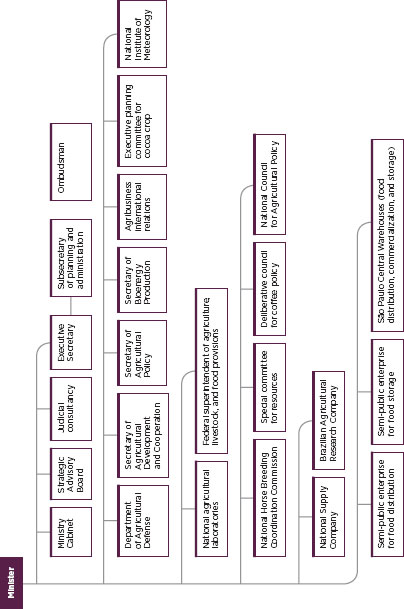
FIGURE C-2
Organization of the Brazilian food regulatory system.

FIGURE C-3
Organization of Indian food regulation.
SOURCE: USDA.
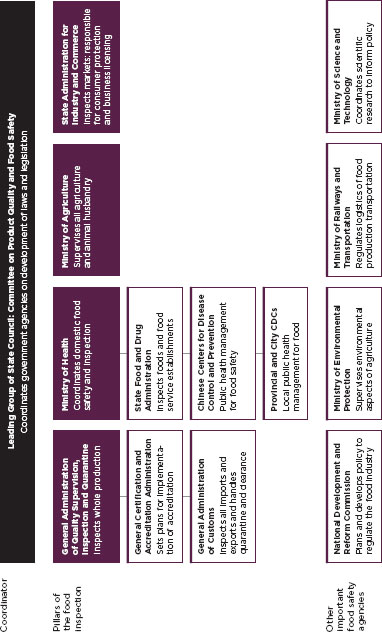
FIGURE C-4
Organization of Chinese food regulatory system.
SOURCE: Broughton and Walker, 2010.
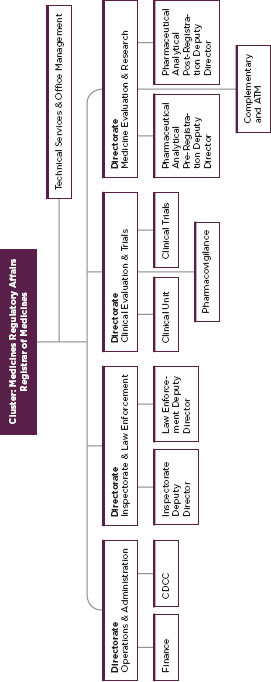
FIGURE C-5
Organization of South African medical product regulatory system.
SOURCE: Government of South Africa.
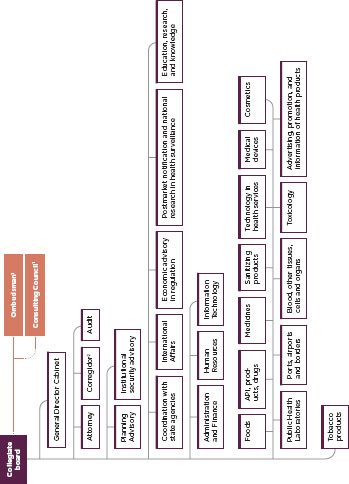
FIGURE C-6
Organization of the Brazilian medical product regulatory system, ANVISA
SOURCE: ANVISA
Notes:
1. The ombudsman and consulting council are not subordinate to the collegiate board.
2. The Corregidor enforces laws and regulations within Anvisa.
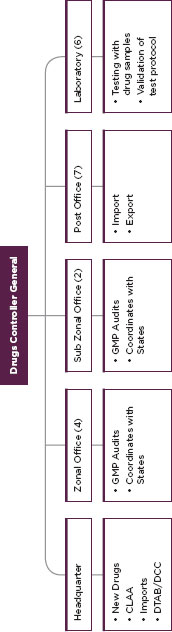
FIGURE C-7
Indian drug control system, the Central Drugs Standard Control Organization.
SOURCE: Government of India.
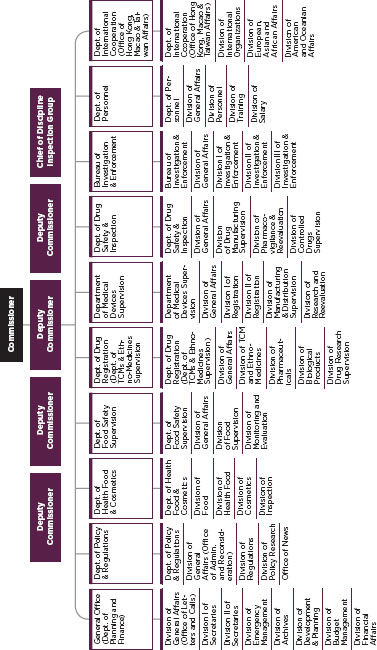
FIGURE C-8
Chinese drug regulatory system, State Food and Drug Administration (SFDA).
SOURCE: SFDA, 2011.
This page intentionally left blankd.









